Filed pursuant to Rule 424(b)(3)
Registration No. 333-239858
PROSPECTUS
31,924,929 Shares
Common Stock
This
prospectus covers resales from time to time by the selling
stockholders named under “Selling Stockholders” in this
prospectus (collectively, the “Selling
Stockholders”) of up to 31,924,929 shares of common stock,
par value $0.001 per share (the “common stock”), of GT
Biopharma, Inc., a Delaware corporation (the
“Company”), that (a) were issued to a Selling
Stockholder pursuant to the terms of a consulting agreement or (b)
that may be issued to certain of the Selling Stockholders either
(i) upon conversion of the Applicable Notes (as defined herein)
issued by the Company in certain private placement transactions, or
(ii) at the option of the Selling Stockholders as holders of the
Applicable Notes, in lieu of cash payments of interest on the
Applicable Notes based upon the then current conversion price for
the Applicable Notes (collectively, the “Registered
Shares”).
The Selling Stockholders may sell the Registered Shares at fixed
prices, at prevailing market prices at the time of the sale, at
varying prices determined at the time of sale, or at negotiated
prices, including, without limitation, in one or more transactions
that may take place by ordinary brokerage transactions,
privately-negotiated transactions or through sales to one or more
underwriters or broker-dealers for resale. See
“Plan
of Distribution.”
All of the Registered Shares sold pursuant to this prospectus will
be offered and sold by the Selling Stockholders. We will not
receive any proceeds from such sales. See
“Use
of Proceeds.” We will
pay all expenses incident to
the registration of the Registered Shares under the Securities Act
of 1933, as amended (the “Securities Act”).
Our common stock is quoted on
the OTCQB, one of the OTC
Markets Group over-the-counter markets, under the trading symbol
“GTBP.” On July 28, 2020, the closing sale price for
our common stock was
$0.18.
The purchase of our
common stock involves a high degree of
risk. You should carefully review and consider
“Risk Factors”
beginning on page 9 of this prospectus and any risks described in
any accompanying prospectus supplement.
Neither the Securities and Exchange
Commission (the “SEC”) nor any state securities commission
has approved or disapproved of these securities or determined if
this prospectus is truthful or complete. Any representation to the
contrary is a criminal offense.
The date of this prospectus is July 28, 2020.
TABLE OF CONTENTS
|
CAUTIONARY NOTICE REGARDING FORWARD-LOOKING
STATEMENTS
|
iii
|
|
|
|
|
ABOUT THIS PROSPECTUS
|
iv
|
|
|
|
|
PROSPECTUS SUMMARY
|
1
|
|
|
|
|
RISK FACTORS
|
8
|
|
|
|
|
USE OF PROCEEDS
|
26
|
|
|
|
|
MARKET INFORMATION
|
26
|
|
|
|
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
|
27
|
|
|
|
|
DESCRIPTION OF BUSINESS
|
34
|
|
|
|
|
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE
|
44
|
|
|
|
|
EXECUTIVE COMPENSATION
|
45
|
|
|
|
|
VOTING SECURITIES AND PRINCIPAL HOLDERS
|
47
|
|
|
|
|
SELLING STOCKHOLDERS
|
49
|
|
|
|
|
PLAN OF DISTRIBUTION
|
51
|
|
|
|
|
DESCRIPTION OF CAPITAL STOCK
|
53
|
|
|
|
|
LEGAL MATTERS
|
56
|
|
|
|
|
EXPERTS
|
56
|
|
|
|
|
WHERE YOU CAN FIND MORE INFORMATION
|
56
|
|
|
|
|
INDEX TO FINANCIAL STATEMENTS
|
F-1
|
CAUTIONARY NOTICE REGARDING FORWARD-LOOKING
STATEMENTS
Some of
the statements in this prospectus are “forward-looking
statements” within the meaning of the safe harbor from
liability established by the Private Securities Litigation Reform
Act of 1995. Forward-looking statements include statements
regarding our current beliefs, goals and expectations about matters
such as our expected financial position and operating results, our
business strategy and our financing plans. The forward-looking
statements in this prospectus are not based on historical facts,
but rather reflect the current expectations of our management
concerning future results and events. The forward-looking
statements generally can be identified by the use of terms such as
“believe,” “expect,”
“anticipate,” “intend,” “plan,”
“foresee,” “may,” “guidance,”
“estimate,” “potential,”
“outlook,” “target,”
“forecast,” “likely” or other similar words
or phrases. Similarly, statements that describe our objectives,
plans or goals are, or may be, forward-looking statements.
Forward-looking statements involve known and unknown risks,
uncertainties and other factors that may cause our actual results,
performance or achievements to be different from any future
results, performance and achievements expressed or implied by these
statements. We cannot guarantee that our forward-looking statements
will turn out to be correct or that our beliefs and goals will not
change. Our actual results could be very different from and worse
than our expectations for various reasons. You should review
carefully all information, including the discussion under
“Risk Factors”
and “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” in this prospectus or under similar
headings in any accompanying prospectus supplement. Any
forward-looking statements in this prospectus are made only as of
the date hereof and, except as may be required by law, we do not
have any obligation to publicly update any forward-looking
statements contained in this prospectus to reflect subsequent
events or circumstances.
This prospectus is part of a registration statement on Form S-1
that we filed with the SEC utilizing a “shelf”
registration process. Under this shelf registration process, the
Selling Stockholders may offer from time to time the Registered
Shares in one or more transactions. The registration statement of
which this prospectus is a part is being filed in accordance with
certain registration rights agreements (collectively, the
“Registration Rights
Agreements”), each by and among the Company and the
applicable Selling Stockholders party thereto. Pursuant to the
Registration Rights Agreements, we have agreed to indemnify and
hold harmless, to the extent permitted by law, each of the Selling
Stockholders party to the such Registration Rights Agreement and
each of such Selling Stockholder’s directors, officers,
partners, members, employees, agents, representatives of and each
other person, if any, who controls such Selling Stockholder within
the meaning of the Securities Act from and against certain losses,
claims, damages and liabilities, including certain liabilities
under the Securities Act.
At the time the Selling Stockholder offers shares of our
common stock registered by this
prospectus, if required, we will provide a prospectus supplement
that will contain specific information about the terms of the
offering and that may add to or update the information in this
prospectus. If the information in this prospectus is inconsistent
with a prospectus supplement, you should rely on the information in
that prospectus supplement. You should read this prospectus and any
applicable prospectus supplement or free writing prospectus, as
well as any post-effective amendments to the registration statement
of which this prospectus forms a part, together with the additional
information described under “Where You Can Find More
Information” before you
make any investment decision.
We are responsible for the information contained in this
prospectus, any applicable prospectus supplement or in any free
writing prospectus prepared by or on behalf of us that we have
referred to you. Neither we nor the Selling Stockholders have
authorized anyone to provide you with additional information or
information different from that contained in this prospectus or in
any applicable prospectus supplement or free writing prospectus
filed with the SEC, and we take no responsibility for any other
information that others may give you. The Selling Stockholder are
offering to sell, and seeking offers to buy, shares of our
common stock only in jurisdictions
where offers and sales are permitted. The information contained in
this prospectus is accurate only as of the date of this prospectus,
regardless of the time of delivery of this prospectus or of any
sale of shares of our common
stock. Our business, operating results or financial condition may
have changed since such date.
No action is being taken in any jurisdiction outside the United
States to permit a public offering of common stock or possession or distribution of this
prospectus in that jurisdiction. Persons who come into possession
of this prospectus in jurisdictions outside the United States are
required to inform themselves about and to observe any restrictions
as to this offering and the distribution of this prospectus
applicable to those jurisdictions.
Unless otherwise indicated, information contained in this
prospectus concerning our industry and the markets in which we
operate, including our general expectations and market position,
market opportunity and market share, is based on information from
our own management estimates and research, as well as from industry
and general publications and research, surveys and studies
conducted by third parties. Management estimates are derived from
publicly available information, our knowledge of our industry and
assumptions based on such information and knowledge, which we
believe to be reasonable. In addition, assumptions and estimates of
our and our industry’s future performance are necessarily
subject to a high degree of uncertainty and risk due to a variety
of factors, including those described in “Risk
Factors.” These and other
factors could cause our future performance to differ materially
from our assumptions and estimates. See “Cautionary Notice Regarding
Forward-Looking Statements.”
This prospectus contains summaries of certain provisions contained
in some of the documents described herein, but reference is made to
the actual documents for complete information. All of the summaries are qualified in their
entirety by the actual documents. Copies of some of the documents
referred to herein have been, or will be, filed or incorporated by
reference as exhibits to the registration statement of which this
prospectus is a part, and you may obtain copies of those documents
as described below under the heading “Where You Can Find More
Information.”
All product and
company names are trademarks of their
respective owners. Solely for convenience, trademarks and trade
names referred to in this prospectus, including logos, artwork and
other visual displays, may appear without the ® or
TM
symbols, but such references are not
intended to indicate, in any way, that their respective owners will
not assert, to the fullest extent under applicable law, their
rights thereto. We do not intend our use or display of other
companies’ trade names or
trademarks to imply a relationship with, or endorsement or
sponsorship of us by, any other companies.
Throughout this prospectus, the terms “we,”
“us,” “our,” and “our Company”
and “the Company” refer to GT Biopharma, Inc., a
Delaware corporation, and/or its related subsidiaries, as the
context may require.
|
|
PROSPECTUS SUMMARY
This summary highlights certain
information about us, this offering and selected information
contained elsewhere in this prospectus. Because this is only a
summary, it does not contain all of the information that may be important to
you or that you should consider before investing in our
common stock. You should read the
entire prospectus carefully, especially the information under
“Risk Factors” set
forth in this prospectus and the information included in any
prospectus supplement or free writing prospectus that we have
authorized for use in connection with this offering. This
prospectus contains forward-looking statements, based on current
expectations and related to future events and our future financial
performance, that involve risks and uncertainties. Our actual
results may vary materially from those discussed in the
forward-looking statements as a result of various factors,
including, without limitation, those set forth under
“Risk Factors,” as
well as other matters described in this prospectus. See
“Cautionary Notice
Regarding Forward-Looking Statements.”
Overview
We are a clinical stage biopharmaceutical company focused on the development and
commercialization of novel immuno-oncology products based off our proprietary
Tri-specific Killer Engager
(TriKE™) and
Tetra-specific Killer Engager
(TetraKE™). Our
TriKE and TetraKE platforms generate
proprietary therapeutics designed to harness and enhance the cancer
killing abilities of a patient’s own natural killer cells
(“NK cells”). Once
bound to an NK cell, our moieties are designed to enhance the NK
cell, and precisely direct it to one or more specifically-targeted
proteins expressed on a specific type of cancer cell or virus
infected cell, ultimately resulting in the targeted cell’s
death. TriKEs and TetraKEs are
made up of recombinant fusion proteins, can be designed to target
any number of tumor antigens on hematologic malignancies, sarcomas
or solid tumors and do not require patient-specific
customization.
We are using our TriKE and
TetraKE platforms with the intent to bring to market
immuno-oncology products that
can treat a range of hematologic malignancies, sarcoma and solid
tumors. The platforms are scalable, and we are putting processes in
place to be able to produce investigational new drug application
(“IND”) ready moieties in a timely manner after a
specific TriKE or TetraKE
conceptual design. After conducting market and competitive
research, specific moieties can then be advanced into the clinic on
our own or through potential collaborations with larger
companies. We are also evaluating, in
conjunction with our Scientific
Advisory Board, additional moieties designed to target different
tumor antigens. We believe our TriKEs and TetraKEs may have the ability, if
approved for marketing, to be used on a stand-alone basis, augment
the current monoclonal antibody therapeutics, be used in
conjunction with more traditional cancer therapy and potentially
overcome certain limitations of current chimeric antigen receptor
(“CAR-T”) therapy.
We are also using our TriKE and
TetraKE platforms to develop therapeutics useful for the treatment
of infectious disease such as for the treatment of patients
infected by the human immunodeficiency virus (“HIV”).
While the use of anti-retroviral drugs has substantially improved
the health and increased the longevity of individuals infected with
HIV, these drugs are designed to suppress virus replication to help
modulate progression to AIDS and to limit further transmission of
the virus. Despite the use of anti-retroviral drugs, infected
individuals retain reservoirs of latent HIV-infected cells that,
upon cessation of anti-retroviral drug therapy, can reactivate and
reestablish an active HIV infection. For a curative therapy,
destruction of these latent HIV infected cells must take place.
The HIV-TriKE contains the
antigen binding fragment (Fab)
from a broadly-neutralizing antibody targeting the
HIV-Env protein. The
HIV-TriKE is designed to target HIV
while redirecting NK cell killing specifically to actively
replicating HIV infected cells. The HIV-TriKE induced NK cell proliferation and
demonstrated the ability in vitro to reactivate and kill
HIV-infected T-cells. These findings indicate a potential role for
the HIV-TriKE in the
reactivation and elimination of the latently infected HIV reservoir
cells by harnessing the NK cell’s ability to mediate the
antibody-directed cellular cytotoxicity
(“ADCC”).
Our initial work has been conducted in collaboration with
the Masonic Cancer Center at
the University of Minnesota under a program led by Dr. Jeffrey
Miller, the Deputy Director.
Dr. Miller is a recognized leader in the field of NK cell and IL-15
biology and their therapeutic potential. We have exclusive rights
to the TriKE and TetraKE
platforms and are generating additional intellectual property
around specific moieties.
Immuno-Oncology Product Candidates
GTB-3550
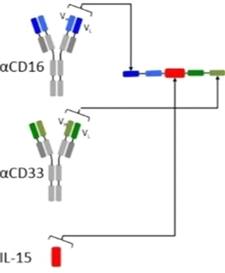
GTB-3550 is our first TriKE product
candidate. It is a tri-specific single-chain variable fragment
(“scFV”)
recombinant fusion protein conjugate composed of the variable
regions of the heavy and light chains of anti-CD16 and anti-CD33
antibodies and a modified form of IL-15. We intend to study this
anti-CD16-IL-15-anti-CD33 TriKE
in CD33 positive leukemias, a marker expressed on tumor cells in
acute myelogenous leukemia (“AML”), myelodysplastic
syndrome (“MDS”) and other hematopoietic malignancies.
CD33 is primarily a myeloid differentiation antigen with endocytic
properties broadly expressed on AML blasts and, possibly, some
leukemic stem cells. CD33 or Siglec-3 (sialic acid binding Ig-like lectin 3, SIGLEC3, SIGLEC3, gp67, p67) is
a transmembrane receptor expressed on cells of myeloid lineage. It
is usually considered myeloid-specific, but it can also be found on
some lymphoid cells. The anti-CD33 antibody fragment that will be
used for these studies was derived from the M195 humanized
anti-CD33 scFV and has been
used in multiple human clinical studies. It has been exploited as
target for therapeutic antibodies for many years. We believe the
recent approval of the antibody-drug conjugate gemtuzumab validates
this targeted approach.
|
|
|
|
The GTB-3550 IND will focus on AML. These patients typically
receive frontline therapy, usually chemotherapy, including
cytarabine and an anthracycline, a therapy that has not changed in
over 40 years. About half will have relapses and require
alternative therapies. In addition, MDS incidence rates have
dramatically increased in the population of the United States from
3.3 per 100,000 individuals from 2001-2004 to 70 per 100,000
annually. MDS is especially prevalent in elderly patients that have
a median age of 76 years at diagnosis. The survival of patients
with MDS is poor due to decreased eligibility, as a result of
advanced age, for allogeneic hematopoietic cell transplantation
(Allo-HSCT), the only curative
MDS treatment (Cogle CR.
Incidence and Burden of the Myelodysplastic Syndromes. Curr Hematol
Malig Rep. 2015; 10(3):272-281). We believe GTB-3550 could serve as
a relatively safe, cost-effective and easy-to-use therapy for
resistant/relapsing AML and could also be combined with
chemotherapy as frontline therapy thus targeting the larger
market.
The IND for GTB-3550 was filed in June 2017 by the University of
Minnesota. The U.S. Food and Drug Administration (the
“FDA”) requested that additional preclinical toxicology
be conducted prior to initiating clinical trials. The FDA also
requested some additional information and clarifications on the
manufacturing and clinical packages. The requested additional
information and clarifications were completed and incorporated by
us into the IND in eCTD format.
We filed the IND amendment in June 2018 and announced on November
1, 2018 that we had received notification from the FDA that the IND
was open and the Company was authorized to initiate a
first-in-human Phase I study
with GTB-3550 in AML, MDS and severe mastocytosis. We began
the Phase I clinical trial in
January 2020.
GTB-C3550
GTB-C3550 is a next-generation, follow-on, to our lead
TriKE, GTB-3550. GTB-C3550 contains a
modified CD16 moiety which has improved binding characteristics and
enhanced tumor cell killing based on functional assays and animal
models of AML. Using our platform technology, we substituted the
anti-CD16 scFv arm in GTB-3550 with a novel humanized
single-domain anti-CD16 antibody to create this second-generation
molecule which may have improved functionality. Single-domain
antibodies, such as GTB-C3550, typically have several advantages,
including better stability and solubility, more resistance to pH
changes, can better recognize hidden antigenic sites, lack of a VL
portion thus preventing VH/VL mispairing and are suitable for
construction of larger molecules. GTB-C3550 induced a potent
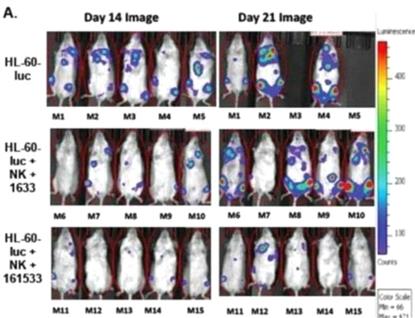
increase in NK cell degranulation, measured by CD107a expression against HL-60 AML tumor targets
when compared to our first-generation TriKE (70.75±3.65% vs. 30.75±5.05%). IFN
production was similarly enhanced (29.2±1.8% vs.
6.55±1.07%). GTB-C3550 also exhibited a robust increase in NK
cell proliferation (57.65±6.05% vs. 20.75±2.55%).
GTB-3550 studies will help inform the development of GTB-C3550
which we expect will de-risk the GTB-C3550 program as data will be
generated to make an informed decision on which, or both, will be
brought into later phase studies.
GTB-1615
GTB-1615 is an example of our first-generation TetraKEs designed for the treatment of solid
tumors. It is a single-chain fusion protein composed of
CD16-IL15-EpCAM-CD133.
EpCAM is found on many solid tumor
cells of epithelial origin and CD133 is a marker for cancer stem
cells. This TetraKE is designed
to target not only the heterogeneous population of cancer cells
found in solid tumors but also the cancer stem cells that are
typically responsible for recurrences. Depending on the
availability of drug supply, we hope to initiate human clinical
testing for certain of our solid tumor product candidates later this
year.
GTB-1550
GTB-1550 is a bispecific scFV recombinant fusion protein-drug
conjugate composed of the variable regions of the heavy and light
chains of anti-CD19 and anti-CD22 antibodies and a modified form of
diphtheria toxin (DT390) as its cytotoxic drug payload. CD19 is a
membrane glycoprotein present on the surface of all stages of B-lymphocyte development and is also
expressed on most B-cell mature lymphoma cells and leukemia cells.
CD22 is a glycoprotein expressed on B-lineage lymphoid precursors,
including precursor ALL (as
defined below), and often is co-expressed with CD19 on mature
B-cell malignancies such as lymphoma.
GTB-1550 targets cancer cells expressing the CD19 receptor or CD22
receptor or both receptors. When GTB-1550 binds to cancer cells,
the cancer cells internalize GTB-1550 and are killed due to the
action of drug’s cytotoxic diphtheria toxin payload. GTB-1550
has completed a Phase I human
clinical trial in patients with relapsed/refractory B-cell lymphoma
or leukemia.
The initial Phase I study
enrolled 25 patients with mature or precursor B-cell lymphoid
malignancies expressing the CD19 receptor or CD22 receptor or both
receptors. All 25 patients
received at least a single course of therapy. The treatment at the
higher doses produced objective tumor responses with one patient in
continuous partial remission and the second in complete remission.
A Phase I/II trial of GTB-1550
in 18 patients was recently completed in patients with
Non-Hodgins Lymphoma
(“NHL”)/Acute
Lymphoblastic Leukemia (“ALL”). The FDA-approved clinical trial was
conducted at the University of
Minnesota’s Masonic Cancer Center and concluded in March
2018. Preliminary data assessment was made in August 2018, and
final assessment made June 24, 2020. Based on the lack of efficacy
demonstrated by GTB-1550 in patients evaluated in two
Phase I/II clinical trials (NHL, ALL
and chronic lymphocytic leukemia (“CLL”)), a decision
has been made to terminate further development. We are currently
evaluating other options for GTB-1550.
|
|
|
|
Recent Developments
Financings
July 2020 Financing
On July 7, 2020, we entered into a securities purchase agreement with ten purchasers pursuant to
which we issued convertible notes in an aggregate principal amount of
approximately $3.2 million (collectively, the “July 2020
Notes”).
The July 2020 Notes are convertible at any time, at the
holder’s option, into shares of our common stock at an initial conversion price of $0.20 per
share, subject to certain
beneficial ownership limitations (with a maximum ownership limit of
9.99%). The
conversion
price is also subject to adjustment due to certain events,
including stock dividends, stock splits and in connection with the
issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
The July 2020 Notes will be subject to mandatory conversion in the
event of the completion of a future financing in the amount of at
least $15 million at a conversion price equal to the lesser of (i)
the conversion price in effect
for the July 2020 Notes on the date of completion of such financing
or (ii) 75% of the lowest per share price at which
common stock may be issued in
connection with any conversion rights associated with the
financing, in each case, subject to the beneficial ownership
limitations described above.
The July 2020 Notes each have a term of six months and mature on
January 7, 2021, unless earlier converted or repurchased. The July
2020 Notes accrue interest at a rate of 10% per annum, subject to
increase to 18% per annum upon and during the occurrence of an
event of default. Interest is payable in cash or, at the
holder’s option, in shares of common stock based on the conversion price then in effect. We may not prepay
the July 2020 Notes without the prior written consent of the
applicable holder.
May 2020 Financing
Between April 20, 2020 and May 7, 2020, we entered into
securities purchase agreements
with eight purchasers pursuant to which we issued
convertible notes in an
aggregate principal amount of approximately $2.0 million
(collectively, the “May 2020
Notes”).
The May 2020 Notes are convertible at any time, at the
holder’s option, into shares of our common stock at an initial conversion price of $0.20 per
share, subject to certain
beneficial ownership limitations (with a maximum ownership limit of
9.99%). The
conversion
price is also subject to adjustment due to certain events,
including stock dividends, stock splits and in connection with the
issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
The May 2020 Notes will be subject to mandatory conversion in the
event of the completion of a future financing in the amount of at
least $15 million at a conversion price equal to the lesser of (i)
the conversion price in effect
for the May 2020 Notes on the date of completion of such financing
or (ii) 75% of the lowest per share price at which
common stock may be issued in
connection with any conversion rights associated with the
financing, in each case, subject to the beneficial ownership
limitations described above.
The May 2020 Notes each have a term of six months and mature
between October 20, 2020 and November 7, 2020, unless earlier
converted or repurchased. The May 2020 Notes accrue interest at a
rate of 10% per annum, subject to increase to 18% per annum upon
and during the occurrence of an event of default. Interest is
payable in cash or, at the holder’s option, in shares
of common stock based on
the conversion price then in
effect. We may not prepay the May 2020 Notes without the prior
written consent of the applicable holder.
January 2020 Financing
On January 30, 2020, we entered into a securities
purchase agreement with one purchaser
pursuant to which we issued convertible notes in an aggregate principal amount of $0.2
million (the “January 2020 Notes”).
The January 2020 Notes are convertible at any time, at the
holder’s option, into shares of our common stock at an initial conversion price of $0.20 per
share, subject to certain
beneficial ownership limitations (with a maximum ownership limit of
9.99%). The
conversion
price is also subject to adjustment due to certain events,
including stock dividends, stock splits and in connection with the
issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
|
|
|
|
The January 2020 Notes have a term of eight months and mature on
September 30, 2020, unless earlier converted or repurchased. The
January 2020 Notes accrue interest at a rate of 10% per annum,
subject to increase to 18% per annum upon and during the occurrence
of an event of default. Interest is payable in cash or, at the
holder’s option, in shares of common stock based on the conversion price then in effect. We may not prepay
the January 2020 Notes without the prior written consent of the
holder.
The January 2020 Notes, together with the July 2020 Notes, the May
2020 Notes and the $0.2 million aggregate principal amount of
convertible notes issued in
December 2019 (the “December 2019 Notes”) pursuant to a
securities purchase agreement,
dated December 19, 2019, between the Company and one purchaser, are
referred to herein as the “Applicable Notes.” For more information
about the December 2019 Notes, see Note 4 to our audited financial
statements, Debt.
For additional information regarding the terms of our
convertible notes and
debentures, including the Applicable
Notes, and the securities purchase agreements pursuant to which they were
issued, see “Management’s Discussion
and Analysis of Financial Condition and Results of
Operations—Indebtedness—Convertible
Notes/Debentures.”
Certain of the Registration Rights Agreements were executed in
connection with the issuance of the Applicable Notes and the
registration statement of which this prospectus is a part is being
filed to fulfill our obligations under such agreements.
Forbearance Agreements
Effective as of June 23, 2020, we entered into Standstill and
Forbearance Agreements (collectively, the “Forbearance
Agreements”) with the holders of approximately $13.2 million
aggregate principal amount of our outstanding convertible
notes
and debentures
(collectively, the “Default Notes”), which are
currently in default. Pursuant to the Forbearance Agreements, the
holders of the Default Notes have agreed to forbear from exercising
their rights and remedies under the Default Notes (including
declaring such Default Notes (together with default amounts and
accrued and unpaid interest) immediately due and payable) until the
earlier of (i) the date that we complete a future financing in the
amount of at least $15 million and, in connection therewith,
commences listing on NASDAQ (collectively, the “New
Financing”) or (ii) October 1, 2020 (the “Termination
Date”).
Pursuant to the Forbearance Agreement, the holders of the Default
Notes have also agreed that the Default Notes (together with
default amounts and accrued and unpaid interest) will be converted
into common stock upon the
closing of a New Financing at a conversion price equal
to the lesser of (i) the conversion price in
effect for the Default Notes on the date of such New Financing or
(ii) 75% of the lowest per share price at
which common stock is or may
be issued in connection with such New Financing, in each case,
subject to certain beneficial ownership limitations (with a maximum
ownership limit of 9.99%). Shares of our
Series J-1
preferred stock (the “Series J-1 Preferred Stock”),
which are convertible into the Company’s common stock, will be
issued in lieu of common stock to the
extent that conversion of the Default Notes is prohibited by such
beneficial ownership limitations.
For additional information regarding the terms of the Forbearance
Agreements, see “Management’s Discussion
and Analysis of Financial Condition and Results of
Operations—Indebtedness—Forbearance
Agreements.”
Settlement with Empery Funds
Settlement Agreement
On June 19, 2020, we entered into a settlement agreement (the
“Settlement
Agreement”) with Empery Asset Master Ltd., Empery Tax
Efficient, LP and Empery Tax Efficient II, LP (collectively, the
“Empery Funds”), Anthony Cataldo and Paul Kessler
resolving all remaining disputes
between the parties pertaining to certain convertible
notes (the
“Original Notes”) and warrants to
purchase common stock, par value
$0.001 per share, of the Company (the “common stock”)
(the “Original
Warrants” and, together with the Original Notes, the
“Original
Securities”) issued by the Company to the Empery Funds in
January 2018 pursuant to a securities purchase agreement. As
previously disclosed, the Empery Funds made various allegations
regarding failures by the Company to take certain actions required
by the terms of the Original
Securities, all of which the
Company denied. See “Description
of Business—Legal Proceedings.”
|
|
|
|
As a result of the Settlement Agreement, the Company paid the
Empery Funds cash payments in an aggregate amount of $0.2 million.
In addition, pursuant to the Settlement Agreement, the Company
issued to the Empery Funds, solely in exchange for the
outstanding Original Securities,
(i) an aggregate of 3.5 million shares of common stock (the
“Settlement Shares”), (ii) pre-funded
warrants to
purchase an aggregate of 5.5 million shares of common stock (the
“Settlement Warrants”) and (iii) senior
convertible notes in an aggregate
principal amount of $0.45 million (the “Settlement
Notes” and,
together with the Settlement Shares and the Settlement
Notes, the
“Settlement
Securities”).
Settlement Notes
The Settlement Notes are convertible
at any time, at the holder’s option, into shares of
common
stock at an initial conversion rate of $0.20 per share, subject to
certain beneficial ownership limitations (with a maximum ownership limit of
4.99%). The
conversion
price is also subject to adjustment due to certain events,
including stock dividends, stock splits and in connection with the
issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
The Settlement Notes have a term of
six months, maturing on December 19, 2020, and bear interest at a
rate of 10% per annum, subject to increase to 18% per annum upon
and during the occurrence of an event of default. Interest is
payable in cash or, at the holder’s option, in shares
of common stock based on
the conversion price then
in effect.
Pursuant to the terms of the Settlement Notes, the Company is
required to make an offer to repurchase, at the holder’s
option, the Settlement Notes at price in cash
equal to 100% of the aggregate principal amount of the
Settlement Note plus accrued and
unpaid interest, if any, to, but excluding, the date of repurchase
following the consummation by the Company of a capital raising
transactions, or a series of transactions, resulting in aggregate
gross proceeds to the Company in excess of $7.5 million. The
Company may not otherwise prepay the Settlement Notes without the prior
written consent of the applicable Empery Funds.
For additional information regarding the terms of the Settlement
Notes and Settlement Agreement, see “Management’s Discussion
and Analysis of Financial Condition and Results of
Operations—Indebtedness— Convertible
Notes/Debentures.”
Settlement Warrants
The Settlement Warrants provide for the purchase of up to an
aggregate of 5.5 million shares of common stock at an
exercise price of $0.20 per share, subject to adjustment in certain
circumstances, and expire on June 19, 2025. Exercise of the
warrant is
subject to certain additional terms and conditions, including
certain beneficial ownership limitations (with a maximum ownership limit of
4.99%).
Collaboration Agreement
On March 10, 2020, we entered into a collaboration
agreement
with Cytovance®
Biologics, a USA-based contract development and manufacturing
organization and a subsidiary of the Shenzhen Hepalink
Pharmaceutical Group Co., Ltd. (“Hepalink”), to provide
development services for a TriKE therapeutic for
the treatment of the coronavirus infection. Under the terms of the
collaboration agreement, the
companies
will focus on preparing sufficient quantities of our
coronavirus TriKE drug
product for
preclinical evaluation using Cytovance’s E.
coli-based Keystone
Expression System™ and
subsequently, will scale-up production using Cytovance’s GMP
microbial manufacturing platform for evaluation of
TriKE in
humans to treat the coronavirus infection.
Corporate Information
Our principal executive offices are located at 9350 Wilshire Blvd.
Suite 203, Beverly Hills, CA 90212, and our telephone number is
(800) 304¬9888. We maintain a website at www.gtbiopharma.com.
Information contained on or accessible through our website is not,
and should not be considered, part of, or incorporated by reference
into, this prospectus.
|
|
|
|
The
Offering
|
|
|
|
Common stock
offered by the Selling Stockholders
|
Up to 31,924,929 shares of our common stock that (a) were issued to a Selling
Stockholder pursuant to the terms of a consulting agreement or (b)
that may be issued to certain of the Selling Stockholders either
(i) upon conversion of the Applicable Notes, or (ii) at the option
of the Selling Stockholders as holders of the Applicable Notes, in
lieu of cash payments of interest on the Applicable Notes based
upon the then current conversion price for the Applicable
Notes.
|
|
|
|
|
|
|
|
|
Use of
Proceeds
|
All of the Registered Shares sold pursuant to this
prospectus will be offered and sold by the Selling Stockholders. We
will not receive any proceeds from such sales. See
“Use
of Proceeds.”
|
|
|
|
|
|
|
|
|
Plan of
Distribution
|
The Selling Stockholders may sell the Registered
Shares at fixed prices, at prevailing market prices at the time of
the sale, at varying prices determined at the time of sale, or at
negotiated prices, including, without limitation, in one or more
transactions that may take place by ordinary brokerage
transactions, privately-negotiated transactions or through sales to
one or more underwriters or broker-dealers for resale. See
“Plan
of Distribution.”
|
|
|
|
|
|
|
|
|
Risk
Factors
|
The purchase of our common stock involves a high degree of risk. You
should carefully review and consider “Risk
Factors” beginning on
page 9 of this prospectus and any risks described in any
accompanying prospectus supplement.
|
|
|
|
|
|
|
|
|
Existing Trading
Market
|
Our common
stock is quoted on the OTCQB, one of the OTC Markets Group over-the-counter markets, under
the trading symbol “GTBP.”
|
|
|
|
|
|
|
|
|
Summary Financial Information
|
|
|
|
The
tables and information below are derived from the Company’s
unaudited consolidated financial statements as of March 31, 2020,
and for the three months ended March 31, 2020 and 2019, and also as
of December 31, 2019.
|
|
Balance Sheet Summary (in thousands)
|
|
|
|
Cash
and cash equivalents
|
$5
|
$28
|
|
Total
assets
|
$295
|
$396
|
|
Total
current liabilities
|
$21,150
|
$19,706
|
|
Total
(deficit) equity
|
$(20,855)
|
$(19,310)
|
Statement of Operations Summary (in thousands except per share
data)
|
|
|
|
Revenue
|
$-
|
$-
|
|
Selling,
general and administrative expenses
|
$746
|
$3,222
|
|
Research
and development
|
$324
|
$834
|
|
Loss
from operations
|
$(1,070)
|
$(4,056)
|
|
Net
loss
|
$(1,708)
|
$(4,510)
|
|
Net
loss per share – basic and diluted
|
$(0.02)
|
$(0.09)
|
|
|
The
tables and information below are derived from the Company’s
audited consolidated financial statements for the years ended
December 31, 2019 and 2019.
|
|
|
Balance Sheet Summary (in
thousands)
|
|
|
|
Cash
and cash equivalents
|
$28
|
$60
|
|
Total
assets
|
$396
|
$25,399
|
|
Total
current liabilities
|
$19,706
|
$14,029
|
|
Total
(deficit) equity
|
$(19,310)
|
$11,370
|
|
Statement of Operations
Summary (in thousands except per share data)
|
|
|
|
|
|
|
|
Revenue
|
$— —
|
$— —
|
|
Selling,
general and administrative expenses
|
$9,790
|
$12,487
|
|
Research
and development
|
$1,667
|
$9,067
|
|
Loss
from operations
|
$(16,056)
|
$(250,069)
|
|
Net
loss
|
$(38,674)
|
$(259,186)
|
|
Net
loss per share – basic and diluted
|
$(0.67)
|
$(5.16)
|
Investing in our common stock involves a high degree of risk. You
should carefully consider the risks and uncertainties described
below in addition to the other information contained in this
prospectus and any prospectus supplement before deciding whether to
invest in shares of our common
stock. If any of the following risks occur, our business, financial
condition or operating results could be harmed. In that case, the
trading price of our common
stock could decline and you may lose part or all of your investment. In the opinion of
management, the risks discussed below represent the material risks
known to us. Additional risks and uncertainties not currently known
to us or that we currently deem immaterial may also impair our
business, financial condition and operating results and adversely
affect the market price of our common stock.
Risks Related to Our Business
Our business is at an early stage of
development and we may not develop therapeutic products that can be
commercialized.
Our business is at an early stage of development. We do not have
immune-oncology products in
late stage clinical trials. We are still in the early stages of
identifying and conducting research on potential therapeutic
products. Our potential
therapeutic products will
require significant research and development and pre-clinical and
clinical testing prior to regulatory approval in the United States
and other countries. We may not be able to obtain regulatory
approvals, enter clinical trials for any of our product candidates or commercialize any
products. Our product candidates may prove to have undesirable
and unintended side effects or other characteristics adversely
affecting their safety, efficacy or cost effectiveness that could
prevent or limit their use. Any product using any of our technology may fail to
provide the intended therapeutic benefits or achieve therapeutic
benefits equal to or better than the standard of treatment at the
time of testing or production.
We have a history of operating losses and we expect to continue to
incur losses for the foreseeable future and we may never generate
revenue or achieve profitability.
As of March 31, 2020, we had an accumulated deficit of $569
million. We have not generated any significant revenue to date, are
not profitable and have incurred losses in each year since our
inception. We do not expect to generate any product sales or royalty revenues for at least
four years. We expect to incur significant additional operating
losses for the foreseeable future as we expand research and
development and clinical trial efforts.
Our ability to achieve long-term profitability is dependent upon
obtaining regulatory approvals for our products and successfully commercializing
our products alone or with
third parties, of which there can be no assurances. However, our
operations may not be profitable even if any of our
products under development are
successfully developed and produced and thereafter commercialized.
Even if we achieve profitability in the future, we may not be able
to sustain profitability in subsequent periods.
Even if we succeed in commercializing one or more of our
product candidates, we expect to
continue to incur substantial research and development and other
expenditures to develop and market additional product candidates. The size of our future net
losses will depend, in part, on the rate of future growth of our
expenses and our ability to generate revenue. Our prior losses and
expected future losses have had and will continue to have an
adverse effect on our stockholders’ equity and working
capital.
Our independent auditor’s report for the years ended December
31, 2019 and 2018 is qualified as to our ability to continue as a
going concern.
Due to the uncertainty of our ability to meet our current operating
and capital expenses, in our audited consolidated financial
statements for the years ended December 31, 2019 and 2018, our
independent auditors included a note to our consolidated financial statements
regarding our ability to continue as a going concern. Recurring
losses from operations and the dependence upon our ability to meet
future financing needs and succeed in our future operations in
order to realize a major portion of our assets have raised a
substantial doubt about our ability to continue as a going concern.
The presence of the going concern note to our consolidated financial statements may
have an adverse impact on the relationships we are developing and
plan to develop with third parties as we continue the
commercialization of our products and could make it challenging and
difficult for us to raise additional financing, all of which could have a material adverse impact
on our business and prospects.
We will need additional capital to
conduct our operations and develop our products, and our ability to obtain the necessary
funding is uncertain.
We have used a significant amount of cash since inception to
finance the continued development and testing of our
product candidates, and we expect to
need substantial additional capital resources in order to develop
our product candidates going
forward and to launch and commercialize any product candidates for which we receive regulatory
approval.
We may not be successful in generating and/or maintaining operating
cash flow, and the timing of our capital expenditures and other
expenditures may not result in cash sufficient to sustain our
operations through the next 12 months. If financing is not
sufficient and additional financing is not available, or available
only on terms that are detrimental to our long-term survival, it
could have a material adverse effect on our ability to continue as
a going concern. The timing and degree of any future capital
requirements will depend on many factors, including:
●
the accuracy of the
assumptions underlying our estimates for capital needs in 2020 and
beyond;
●
scientific and
clinical progress in our research and development
programs;
●
the magnitude and
scope of our research and development programs and our ability to
establish, enforce and maintain strategic arrangements for
research, development, clinical testing, manufacturing and
marketing;
●
our progress with
pre-clinical development and clinical trials;
●
the time and costs
involved in obtaining regulatory approvals;
●
the costs involved
in preparing, filing, prosecuting, maintaining, defending and
enforcing patent claims; and
●
the number and type
of product candidates that we pursue.
Additional financing through strategic collaborations, public or
private equity or debt financings or other financing sources may
not be available on acceptable terms, or at all. The completion of financings involving the
issuance of additional common
stock or other securities convertible into, or exchangeable
for, common stock (such
as warrants or additional
convertible notes) could also
result in significant dilution to our
stockholders.
Further, if we obtain additional funds through arrangements
with collaborative partners, these arrangements may require us to
relinquish rights to some of our technologies, product candidates or products that we would otherwise seek to develop
and commercialize on our own.
If sufficient capital is not available, we may be required to
delay, reduce the scope of or eliminate one or more of our research
or product development
initiatives, any of which could have a material adverse effect on
our financial condition or business prospects.
Our current and future indebtedness may impose significant
operating and financial restrictions on us and affect our ability
to access liquidity.
As of the date of this prospectus, after giving effect to (i) the
issuance of the July 2020 Notes and the May 2020 Notes and (ii) the
issuance of the Settlement Notes pursuant to the Settlement
Agreement (but excluding the impact of any conversions of our
convertible notes after the filing date of the registration
statement of which this prospectus forms a part), we had
approximately $18.8 million aggregate principal amount of
convertible notes and
debentures outstanding, a portion of
which are secured by a first priority security interest in
substantially all of the assets
of the Company and its subsidiaries. Our existing
convertible notes and
debentures do, and any future
instruments governing our indebtedness may, contain a number of
restrictive covenants that impose significant operating and
financial restrictions on us. For example, our existing
convertible notes and
debentures include restrictions on our
ability to, among other things:
●
incur
additional indebtedness;
●
place
liens on our or our subsidiaries’ assets;
●
repurchase shares of our common stock or repay existing
indebtedness;
●
pay
cash dividends or distributions on our equity
securities;
●
engage
in certain fundamental change transactions; and
●
engage
in transactions with affiliates.
A failure by us or our subsidiaries to comply with the covenants
and restrictions contained in the agreements governing our indebtedness could result
in an event of default under such indebtedness, which could
adversely affect our ability to respond to changes in our business
and manage our operations. Upon the occurrence of an event of
default under any of the agreements governing our indebtedness, the holders
could elect to declare all
amounts outstanding to be due and payable and exercise other
remedies as set forth in the agreements. Further, an event of default or
acceleration of indebtedness under one instrument may constitute an
event of default—or cross-default—under another
instrument. For example, in June 2020, we entered into the
Forbearance Agreements with holders of the Default Notes pursuant
to which such holders have agreed forbear from exercising
their rights and remedies under the Default
Notes (including declaring
such Default Notes (together with default amounts and accrued and
unpaid interest) immediately due and payable) for a specified
period of time.
If any of our indebtedness (including the Default Notes) were to be
accelerated, there can be no assurance that our assets would be
sufficient to repay this indebtedness in full, which could have a
material adverse effect on our ability to continue to operate as a
going concern.
The cost of our research and
development programs may be significantly higher than expected and
there is no assurance that they will successful in a timely manner,
or at all.
Our currently projected expenditures for 2020 include approximately
$12 million to $15 million for research and development. The actual
cost of our programs could differ significantly from our current
projections if we change our planned development process. In the
event that actual costs of our clinical program, or any of our
other ongoing research activities, are significantly higher than
our current estimates, we may be required to significantly modify
our planned level of operations.
The successful development of any product candidate is highly uncertain. It is
difficult to reasonably estimate or know the nature, timing and
costs of the efforts necessary to complete the development of, or
the period in which material net cash inflows are expected to
commence from any product
candidate, due to the numerous risks and uncertainties associated
with developing and commercializing drugs. Any failure to complete
any stage of the development of products in a timely manner could have a material
adverse effect on our operations, financial position and
liquidity.
We have identified material weaknesses
in our internal controls over financial reporting and have not yet
remedied these weaknesses. If we fail to maintain an effective
system of internal control over financial reporting, we may not be
able to accurately report our financial results or prevent fraud.
As a result, stockholders could lose confidence in our financial
and other public reporting, which would harm our business and the
trading price of our common
stock.
Effective internal control over financial reporting is necessary
for us to provide reliable financial reports and, together with
adequate disclosure controls and procedures, are designed to
prevent fraud. Any failure to implement required new or improved
controls, or difficulties encountered in their implementation,
could cause us to fail to meet our reporting obligations.
Ineffective internal control could also cause investors to lose
confidence in our reported financial information, which could have
a negative effect on the trading price of our common stock.
We have identified material weaknesses in our internal control over
financial reporting as a company. As defined in Regulation 12b-2 under the
Securities Exchange Act of 1934, as amended (the
“Exchange Act”), a
“material weakness” is a deficiency, or combination of
deficiencies, in internal control over financial reporting, such
that there is a reasonable possibility that a material misstatement
of our annual or interim consolidated financial statements will not
be prevented, or detected on a timely basis. Specifically, we
determined that we had the following material weaknesses in our
internal control over financial reporting as of December 31, 2019:
(i) inadequate segregation of duties; (ii) risks of executive
override; and (iii) insufficient written policies and procedures
for accounting and financial reporting with respect to the
requirements and application of both generally accepted accounting
principles in the United States of America
(“GAAP”) and SEC
regulations.
As of the date of this prospectus, we have not remediated these
material weaknesses. We are taking steps, and intend to take
additional steps, to mitigate the issues identified and implement a
functional system of internal controls over financial reporting.
Such measures will include, but not be limited to: hiring of
additional employees in our finance and accounting department,
although the timing of such hires is largely dependent on our
securing additional financing to cover such costs; preparation of
risk-control matrices to identify key risks and develop and
document policies to mitigate those risks; and identification and
documentation of standard operating procedures for key financial
and SEC reporting activities. The implementation of these
initiatives may not fully address any material weakness or other
deficiencies that we may have in our internal control over
financial reporting.
Even if we develop effective internal control over financial
reporting, such controls may become inadequate due to changes in
conditions or the degree of compliance with such policies or
procedures may deteriorate, which could result in the discovery of
additional material weaknesses and deficiencies. In any event, the
process of determining whether our existing internal control over
financial reporting is compliant with Section 404 of the
Sarbanes-Oxley Act (“Section 404”) and sufficiently
effective requires the investment of substantial time and
resources, including by certain members of our senior management.
As a result, this process may divert internal resources and take a
significant amount of time and effort to complete. In addition, we
cannot predict the outcome of this process and whether we will need
to implement remedial actions in order to establish effective
controls over financial reporting. The determination of whether or
not our internal controls are sufficient and any remedial actions
required could result in us incurring additional costs that we did
not anticipate, including the hiring of outside consultants. We may
also fail to timely complete our evaluation, testing and any
remediation required to comply with Section 404.
We are required, pursuant to Section 404, to furnish a report by
management on, among other things, the effectiveness of our
internal control over financial reporting. However, for as long as
we are a “smaller reporting company,” our independent registered public
accounting firm will not be required to attest to the effectiveness
of our internal control over financial reporting pursuant to
Section 404. While we could be a smaller reporting
company for an indefinite amount of
time, and thus relieved of the above-mentioned attestation
requirement, an independent assessment of the effectiveness of our
internal control over financial reporting could detect problems
that our management’s assessment might not. Such undetected
material weaknesses in our internal control over financial
reporting could lead to financial statement restatements and
require us to incur the expense of remediation.
If our efforts to protect the proprietary nature of the
intellectual property related to our technologies are not adequate,
we may not be able to compete effectively in our market and our
business would be harmed.
We rely upon a combination of patents, trade secret protection and
confidentiality agreements to
protect the intellectual property related to our technologies. Any
disclosure to, or misappropriation by, third parties of our trade
secret or other confidential information could enable competitors
to quickly duplicate or surpass our technological achievements,
thus eroding any competitive advantage we may derive from this
intellectual property.
The strength of patents in the biotechnology and pharmaceutical
field involves complex legal and scientific questions and can be
uncertain. The patent applications we own or license may fail to
result in issued patents in the United States or in foreign
countries. Third parties may challenge the validity, enforceability
or scope of any issued patents we own or license or any
applications that may issue as patents in the future, which may
result in those patents being narrowed, invalidated or held
unenforceable. Even if they are unchallenged, our patents and
patent applications may not adequately protect our intellectual
property or prevent others from developing similar
products that do not fall within the
scope of our patents. If the breadth or strength of protection
provided by the patents we hold or pursue is threatened, our
ability to commercialize any product candidates with technology protected by
those patents could be threatened. Further, if we encounter delays
in our clinical trials, the period of time during which we would
have patent protection for any covered product candidates that obtain regulatory approval
would be reduced.
In addition to the protection afforded by patents, we seek to rely
on trade secret protection and confidentiality agreements to protect proprietary know-how that is
not patentable, processes for which patents are difficult to
enforce and any other elements of our discovery platform and drug
development processes that involve proprietary know-how,
information or technology that is not covered by patents or not
amenable to patent protection. Although we require
all of our employees and certain
consultants and advisors to enter into intellectual property
assignment agreements,
and all of our employees,
consultants, advisors and any third parties who have access to our
proprietary know-how, information or technology to enter into
confidentiality agreements, our
trade secrets and other proprietary information may be disclosed or
competitors may otherwise gain access to such information or
independently develop substantially equivalent information.
Further, the laws of some foreign countries do not protect
proprietary rights to the same extent or in the same manner as the
laws of the United States. As a result, we may encounter
significant difficulty in protecting and defending our intellectual
property both in the United States and abroad. If we are unable to
prevent material disclosure of the trade secret intellectual
property related to our technologies to third parties, we may not
be able to establish or maintain the competitive advantage that we
believe is provided by such intellectual property, which could
materially adversely affect our market position and business and
operational results.
Claims that we infringe the intellectual property rights of others
may prevent or delay our drug discovery and development
efforts.
Our research, development and commercialization activities, as well
as any product candidates
or products resulting from
those activities, may infringe or be accused of infringing a patent
or other form of intellectual property under which we do not hold a
license or other rights. Third parties may assert that we are
employing their proprietary technology without authorization. There
may be third-party patents of which we are currently unaware, with
claims that cover the use or manufacture of our product candidates or the practice of our related
methods. Because patent applications can take many years to issue
and remain confidential for a period of time after filing, there
may be currently pending patent applications that may later result
in issued patents that our product candidates may infringe. In addition,
third parties may obtain patents in the future and claim that use
of our technologies infringes one or more claims of these patents.
If our activities or product
candidates infringe the patents or other intellectual property
rights of third parties, the holders of such intellectual property
rights may be able to block our ability to commercialize
such product candidates or
practice our methods unless we obtain a license under the
intellectual property rights or until any applicable patents expire
or are determined to be invalid or
unenforceable.
Defense of any intellectual property infringement claims against
us, regardless of their merit, would involve substantial litigation
expense and would be a significant diversion of employee resources
from our business. In the event of a successful claim of
infringement against us, we may have to pay substantial damages,
obtain one or more licenses from third parties, limit our business
to avoid the infringing activities, pay royalties and/or redesign
our infringing product
candidates or methods, any or all of which may be impossible or require
substantial time and monetary expenditure. Further, if we were to
seek a license from the third party holder of any applicable
intellectual property rights, we may not be able to obtain the
applicable license rights when needed or on commercially reasonable
terms, or at all. The
occurrence of any of the above events could prevent us from
continuing to develop and commercialize one or more of our
product candidates and our business
could materially suffer.
We may desire, or be forced, to seek
additional licenses to use intellectual property owned by third
parties, and such licenses may not be available on commercially
reasonable terms, or at all.
A third party may hold intellectual property, including patent
rights, that are important or necessary to the development of
our product candidates, in
which case we would need to obtain a license from that third party
or develop a different formulation of the product that does not infringe upon the applicable
intellectual property, which may not be possible. Additionally, we
may identify product candidates
that we believe are promising and whose development and other
intellectual property rights are held by third parties. In such a
case, we may desire to seek a license to pursue the development of
those product candidates. Any
license that we may desire to obtain or that we may be forced to
pursue may not be available when needed on commercially reasonable
terms, or at all. Any inability
to secure a license that we need or desire could have a material
adverse effect on our business, financial condition and
prospects.
The patent protection covering some of
our product candidates may be
dependent on third parties, who may not effectively maintain that
protection.
While we expect that we will generally seek to gain the right to
fully prosecute any patents covering product candidates we may in-license from
third-party owners, there may be instances when platform technology
patents that cover our product
candidates remain controlled by our licensors. If any of our
current or future licensing partners that retain the right to
prosecute patents covering the product candidates we license from them fail to
appropriately maintain that patent protection, we may not be able
to prevent competitors from developing and selling competing
products or practicing competing
methods and our ability to generate revenue from any
commercialization of the affected product candidates may suffer.
We may be involved in lawsuits to protect or enforce our patents or
the patents of our licensors, which could be expensive,
time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our current
or potential licensors. To attempt to stop infringement or
unauthorized use, we may need to enforce one or more of our
patents, which can be expensive and time-consuming and distract
management. If we pursue any litigation, a court may decide that a
patent of ours or our licensors is not valid or is unenforceable,
or may refuse to stop the other party from using the relevant
technology on the grounds that our patents do not cover the
technology in question. Further, the legal systems of certain
countries, particularly certain developing countries, do not favor
the enforcement of patents, which could reduce the likelihood of
success of any infringement proceeding we pursue in any such
jurisdiction. An adverse result in any infringement litigation or
defense proceedings could put one or more of our patents at risk of
being invalidated, held unenforceable, or interpreted narrowly and
could put our patent applications at risk of not issuing, which
could limit our ability to exclude competitors from directly
competing with us in the applicable jurisdictions.
Interference proceedings provoked by third parties or brought by
the U.S. PTO may be necessary to determine the priority of
inventions with respect to our patents or patent applications or
those of our licensors. An unfavorable outcome could require us to
cease using the related technology or to attempt to license rights
to use it from the prevailing party. Our business could be harmed
if the prevailing party does not offer us a license on commercially
reasonable terms, or at all.
Litigation or interference proceedings may fail and, even if
successful, may result in substantial costs and distract our
management and other employees.
If we are unsuccessful in obtaining or maintaining patent
protection for intellectual property in development, our business
and competitive position would be harmed.
We are seeking patent protection for some of our technology
and product candidates. Patent
prosecution is a challenging process and is not assured of success.
If we are unable to secure patent protection for our technology
and product candidates, our
business may be adversely impacted.
In addition, issued patents and pending international applications
require regular maintenance. Failure to maintain our portfolio may
result in loss of rights that may adversely impact our intellectual
property rights, for example by rendering issued patents
unenforceable or by prematurely terminating pending international
applications.
If we are unable to protect the confidentiality of our trade
secrets, our business and competitive position would be
harmed.
In addition to seeking patents for some of our technology
and product candidates, we also
rely on trade secrets, including unpatented know-how, technology
and other proprietary information, to maintain our competitive
position. We currently, and expect in the future to continue to,
seek to protect these trade secrets, in part, by entering into
confidentiality agreements with
parties who have access to them, such as our employees,
consultants, advisors and other third parties. We also
require all of our employees
and certain consultants and advisors to enter into intellectual
property assignment agreements.
Despite these efforts, any of these parties may breach the
agreements and disclose our
proprietary information, including our trade secrets, and we may
not be able to obtain adequate remedies for any such disclosure.
Enforcing a claim that a party illegally disclosed or
misappropriated a trade secret is difficult, expensive and
time-consuming, and the outcome is unpredictable. In addition, some
courts inside and outside the United States are less willing or
unwilling to protect trade secrets. If any of our trade secrets
were to be lawfully obtained or independently developed by a
competitor, we would have no right to prevent them, or those to
whom they disclose the trade secrets, from using that technology or
information to compete with us. If any of our trade secrets were to
be disclosed to or independently developed by a competitor, our
competitive position would be harmed.
If we fail to meet our obligations
under our license agreements,
we may lose our rights to key technologies on which our business
depends.
Our business depends in part on licenses from third parties. These
third-party license agreements
impose obligations on us, such as payment obligations and
obligations to diligently pursue development of commercial
products under the licensed patents.
If a licensor believes that we have failed to meet our obligations
under a license agreement, the
licensor could seek to limit or terminate our license rights, which
could lead to costly and time-consuming litigation and,
potentially, a loss of the licensed rights. During the period of
any such litigation, our ability to carry out the development and
commercialization of potential products could be significantly and negatively
affected. If our license rights were restricted or ultimately lost,
our ability to continue our business based on the affected
technology platform could be severely adversely
affected.
We will have to hire additional executive officers and employees to
operate our business. If we are unable to hire qualified personnel,
we may not be able to implement our business strategy.
We currently have only two full-time employees. The loss of the
services of any one of our employees could delay our
product development programs and our
research and development efforts. We do not maintain key person
life insurance on any of our officers, employees, consultants or
advisors. In order to develop our business in accordance with our
business strategy, we will have to hire additional qualified
personnel, including in the areas of manufacturing, clinical trials
management, regulatory affairs, finance and business development.
We will need to raise sufficient funds to hire the necessary
employees and have commenced our search for additional key
employees.
Moreover, there is intense competition for a limited number of
qualified personnel among biopharmaceutical, biotechnology,
pharmaceutical and other businesses. Many of the other
pharmaceutical companies
against which we compete for qualified personnel have greater
financial and other resources, different risk profiles, longer
histories in the industry and greater ability to provide valuable
cash or stock incentives to potential recruits than we do. They
also may provide more diverse opportunities and better chances for
career advancement. Some of these characteristics may be more
appealing to high quality candidates than what we are able to offer
as an early-stage company. If
we are unable to continue to attract and retain high quality
personnel, the rate and success at which we can develop and
commercialize product
candidates will be limited.
We depend on key personnel for our continued operations and future
success, and a loss of certain key personnel could significantly
hinder our ability to move forward with our business
plan.
Because of the specialized nature of our business, we are highly
dependent on our ability to identify, hire, train and retain highly
qualified scientific and technical personnel for the research and
development activities we conduct or sponsor. The loss of one or
more key executive officers, or scientific officers, would be
significantly detrimental to us. In addition, recruiting and
retaining qualified scientific personnel to perform research and
development work is critical to our success. Our anticipated growth
and expansion into areas and activities requiring additional
expertise, such as clinical testing, regulatory compliance,
manufacturing and marketing, will require the addition of new
management personnel and the development of additional expertise by
existing management personnel. There is intense competition for
qualified personnel in the areas of our present and planned
activities. Accordingly, we may not be able to continue to attract
and retain the qualified personnel, which would adversely affect
the development of our business.
We may be subject to claims by third parties asserting that our
employees or we have misappropriated their intellectual property,
or claiming ownership of what we regard as our own intellectual
property.
Many of our employees, consultants and advisors were previously
employed at universities or other biotechnology or
pharmaceutical companies,
including our competitors or potential competitors. Although we try
to ensure that our employees, consultants and advisors do not use
the proprietary information or know-how of others in their work for
us, with contractual provisions and other procedures, we may be
subject to claims that these employees, consultants or advisors
have used or disclosed intellectual property, including trade
secrets or other proprietary information, of any such
employee’s, consultant’s or advisor’s former
employers. Litigation may be necessary to defend against any such
claims.
In addition, while it is our policy to require our employees,
consultants and advisors who may be involved in the development of
intellectual property to execute agreements assigning such intellectual property to
us, we may be unsuccessful in executing such an agreement with each party who in fact contributes
to the development of intellectual property that we regard as our
own. Further, the terms of such assignment agreements may be breached and we may not be able
to successfully enforce their terms, which may force us to bring
claims against third parties, or defend claims they may bring
against us, to determine the ownership of intellectual property
rights we may regard and treat as our own.
Our employees, consultants and advisors may engage in misconduct or
other improper activities, including noncompliance with regulatory
standards and requirements, which could cause our business to
suffer.
We are exposed to the risk of fraud or other misconduct by our
employees, consultants or advisors. Misconduct by employees,
consultants or advisors could include intentional failures to
comply with regulations of governmental authorities, such as the
FDA or the European Medicines Agency (the “EMA”), to
provide accurate information to the FDA or EMA, to comply with
manufacturing standards we have established, to comply with
federal, state and international healthcare fraud and abuse laws
and regulations as they may become applicable to our operations, to
report financial information or data accurately or to disclose
unauthorized activities to us. Such misconduct could also involve
the improper use of information obtained in the course of clinical
trials, which could result in regulatory sanctions and serious harm
to our reputation. It is not always possible to identify and deter
such misconduct, and the precautions we currently take and the
procedures we may establish in the future as our operations and
employee base expand to detect and prevent this type of activity
may not be effective in controlling unknown or unmanaged risks or
losses or in protecting us from governmental investigations or
other actions or lawsuits stemming from a failure by our employees,
consultants or advisors to comply with such laws or regulations. If
any such actions are instituted against us, and we are not
successful in defending ourselves or asserting our rights, those
actions could have a significant impact on our business and results
of operations, including the imposition of significant fines or
other sanctions.
Our reliance on the activities of our
non-employee consultants, research institutions and scientific
contractors, whose activities are not wholly within our control,
may lead to delays in development of our proposed
products.
We rely extensively upon and have relationships with scientific
consultants at academic and other institutions, some of whom
conduct research at our request, and other consultants with
expertise in clinical development strategy or other matters. These
consultants are not our employees and may have commitments to, or
consulting or advisory contracts with, other entities that may
limit their availability to us. We have limited control over the
activities of these consultants and, except as otherwise required
by our collaboration and consulting agreements to the extent they exist,
can expect only limited amounts of their time to be dedicated to
our activities. These research facilities may have commitments to
other commercial and non-commercial entities. We have limited
control over the operations of these laboratories and can expect
only limited amounts of time to be dedicated to our research
goals.
It may take longer to complete our
clinical trials than we project, or we may not be able to complete
them at all.
For budgeting and planning purposes, we have projected the date for
the commencement, continuation and completion of our various
clinical trials. However, a number of factors, including scheduling
conflicts with participating clinicians and clinical institutions,
and difficulties in identifying and enrolling patients who meet
trial eligibility criteria, may cause significant delays. We may
not commence or complete clinical trials involving any of
our products as projected or
may not conduct them successfully.
We expect to rely on medical institutions, academic institutions or
clinical research organizations to conduct, supervise or monitor
some or all aspects of clinical
trials involving our products.
We will have less control over the timing and other aspects of
these clinical trials than if we conducted them entirely on our
own. If we fail to commence or complete, or experience delays in,
any of our planned clinical trials, our stock price and our ability
to conduct our business as currently planned could be
harmed.
Clinical drug development is costly, time-consuming and uncertain,
and we may suffer setbacks in our clinical development program that
could harm our business.
Clinical drug development for our product candidates is costly, time-consuming and
uncertain. Our product
candidates are in various stages of development and while we expect
that clinical trials for these product candidates will continue for several
years, such trials may take significantly longer than expected to
complete. In addition, we, the FDA, an institutional review
board (“IRB”) or other
regulatory authorities, including state and local agencies and
counterpart agencies in foreign countries, may suspend, delay,
require modifications to or terminate our clinical trials at any
time, for various reasons, including:
●
discovery of safety
or tolerability concerns, such as serious or unexpected toxicities
or side effects or exposure to otherwise unacceptable health risks,
with respect to study participants;
●
lack of
effectiveness of any product candidate during clinical trials or
the failure of our product candidates to meet specified
endpoints;
●
delays in subject
recruitment and enrollment in clinical trials or inability to
enroll a sufficient number of patients in clinical trials to ensure
adequate statistical ability to detect statistically significant
treatment effects;
●
difficulty in
retaining subjects and volunteers in clinical trials;
●
difficulty in
obtaining IRB approval for studies to be conducted at each clinical
trial site;
●
delays in
manufacturing or obtaining, or inability to manufacture or obtain,
sufficient quantities of materials for use in clinical
trials;
●
inadequacy of or
changes in our manufacturing process or the product formulation or
method of delivery;
●
delays or failure
in reaching agreement on acceptable terms in clinical trial
contracts or protocols with prospective contract research
organizations (“CROs”), clinical trial sites and other
third-party contractors;
●
inability to add a
sufficient number of clinical trial sites;
●
uncertainty
regarding proper formulation and dosing;
●
failure by us, our
employees, our consultants or advisors, our CROs or their employees
or other third-party contractors to comply with contractual and
applicable regulatory requirements or to perform their services in
a timely or acceptable manner;
●
scheduling
conflicts with participating clinicians and clinical
institutions;
●
failure to design
appropriate clinical trial protocols;
●
inability or
unwillingness of medical investigators to follow our clinical
protocols;
●
difficulty in
maintaining contact with subjects during or after treatment, which
may result in incomplete data; or
●
changes in
applicable laws, regulations and regulatory policies.
If we experience delays or difficulties in the enrollment of
patients in clinical trials, those clinical trials could take
longer than expected to complete and our receipt of necessary
regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for
our product candidates if we
are unable to locate and enroll a sufficient number of eligible
patients to participate in these trials as required by the FDA or
similar regulatory authorities outside the United States. In
particular, because we are focused on patients with molecularly
defined cancers, our pool of suitable patients may be smaller and
more selective and our ability to enroll a sufficient number of
suitable patients may be limited or take longer than anticipated.
In addition, some of our competitors have ongoing clinical trials
for product candidates that
treat the same indications as our product candidates, and patients who would
otherwise be eligible for our clinical trials may instead enroll in
clinical trials of our competitors’ product candidates.
Patient enrollment for any of our clinical trials may also be
affected by other factors, including without
limitation:
●
the severity of the
disease under investigation;
●
the frequency of
the molecular alteration we are seeking to target in the applicable
trial;
●
the eligibility
criteria for the study in question;
●
the perceived risks
and benefits of the product candidate under study;
●
the extent of the
efforts to facilitate timely enrollment in clinical
trials;
●
the patient
referral practices of physicians;
●
the ability to
monitor patients adequately during and after
treatment;
●
the proximity and
availability of clinical trial sites for prospective
patients.
●
unforeseen safety
issues;
●
determination of
dosing issues;
●
inability to
demonstrate effectiveness during clinical trials;
●
slower than
expected rates of patient recruitment;
●
inability to
monitor patients adequately during or after treatment;
and
●
inability or
unwillingness of medical investigators to follow our clinical
protocols.
In
addition, we or the FDA, may suspend our clinical trials at any
time if it appears that we are exposing participants to
unacceptable health risks or if the FDA finds deficiencies in our
IND submissions or the conduct of these trials.
We are subject to extensive
regulation, which can be costly and time consuming and can subject
us to unanticipated delays. even if we obtain regulatory approval
for some of our products,
those products may still face
regulatory difficulties.
All of our potential products, processing and manufacturing activities,
are subject to comprehensive regulation by the FDA in the United
States and by comparable authorities in other countries. The
process of obtaining FDA and other required regulatory approvals,
including foreign approvals, is expensive and often takes many
years and can vary substantially based upon the type, complexity
and novelty of the products
involved. In addition, regulatory agencies may lack experience with
our technologies and products,
which may lengthen the regulatory review process, increase our
development costs and delay or prevent their
commercialization.
If we violate regulatory requirements at any
stage, whether before or after we obtain marketing approval, the
FDA may take enforcement action(s) against us, which could include
issuing a warning or untitled letter, placing a clinical hold on an
ongoing clinical trial, product
seizure, enjoining our operations, refusal to consider our
applications for pre-market approval, refusal of an investigational
new drug application, fines, or even civil or criminal liability,
any of which could materially harm our reputation and financial
results. Additionally, we may not be able to obtain the labeling
claims necessary or desirable for the promotion of our
products. We may also be required to
undertake post-marketing trials to provide additional evidence of
safety and effectiveness. In addition, if we or others identify
side effects after any of our adoptive therapies are on the market,
or if manufacturing problems occur, regulators may withdraw their
approval and reformulations, additional clinical trials, changes in
labeling of our products, and
additional marketing applications may be
required.
Any of the following factors, among others, could
cause regulatory approval for our product candidates to be delayed, limited or
denied:
●
the product
candidates require significant clinical testing to demonstrate
safety and effectiveness before applications for marketing approval
can be filed with the FDA and other regulatory
authorities;
●
data obtained from
pre-clinical and nonclinical animal testing and clinical trials can
be interpreted in different ways, and regulatory authorities may
not agree with our respective interpretations or may require us to
conduct additional testing;
●
negative or
inconclusive results or the occurrence of serious or unexpected
adverse events during a clinical trial could cause us to delay or
terminate development efforts for a product candidate;
and/or
●
FDA and other
regulatory authorities may require expansion of the size and scope
of the clinical trials.
Any difficulties or failures that we encounter in securing
regulatory approval for our product candidates would likely have a substantial
adverse impact on our ability to generate product sales and could make any search for a
collaborative partner more difficult.
Obtaining regulatory approval even after clinical trials that are
believed to be successful is an uncertain process.
Even if we complete our planned clinical trials and believe the
results were successful, obtaining regulatory approval is a
lengthy, expensive and uncertain process, and the FDA or other
regulatory agencies may delay, limit or deny approval of any of our
applications for pre-market approval for many reasons,
including:
●
we may not be able
to demonstrate to the FDA’s satisfaction that our product
candidates are safe and effective for any indication;
●
the results of
clinical trials may not meet the level of statistical significance
or clinical significance required by the FDA for
approval;
●
the FDA may
disagree with the number, design, size, conduct or implementation
of our clinical trials;
●
the FDA may not
find the data from pre-clinical studies and clinical trials
sufficient to demonstrate that the clinical and other benefits of
our product candidates outweigh their safety risks;
●
the FDA may
disagree with our interpretation of data from pre-clinical studies
or clinical trials, or may not accept data generated at our
clinical trial sites;
●
the data collected
from pre-clinical studies and clinical trials of our product
candidates may not be sufficient to support the submission of
applications for regulatory approval;
●
the FDA may have
difficulties scheduling an advisory committee meeting in a timely
manner, or the advisory committee may recommend against approval of
our application or may recommend that the FDA require, as a
condition of approval, additional pre-clinical studies or clinical
trials, limitations on approved labeling, or distribution and use
restrictions;
●
the FDA may require
development of a risk evaluation and mitigation strategy as a
condition of approval;
●
the FDA may
identify deficiencies in the manufacturing processes or facilities
of third-party manufacturers with which we enter into agreements
for clinical and commercial supplies;
●
the FDA may change
their approval policies or adopt new regulations that adversely
affect our applications for pre-market approval; and
●
the FDA may require
simultaneous approval for both adults and for children and
adolescents delaying needed approvals, or we may have successful
clinical trial results for adults but not children and adolescents,
or vice versa.
Before we can submit an application for regulatory approval in the
United States, we must conduct a pivotal, Phase III trial. We will also need to agree on a
protocol with the FDA for a clinical trial before commencing the
trial. Phase III clinical
trials frequently produce unsatisfactory results even though prior
clinical trials were successful. Therefore, even if the results of
our Phase II trials are
successful, the results of the additional trials that we conduct
may or may not be successful. Further, our product candidates may not be approved even if
they achieve their primary endpoints in Phase III clinical trials. The FDA or other
foreign regulatory authorities may disagree with our trial design
and our interpretation of data from preclinical studies and
clinical trials. Any of these regulatory authorities may change
requirements for the approval of a product candidate even after reviewing and
providing comments or advice on a protocol for a clinical trial.
The FDA or other regulatory agencies may require that we conduct
additional clinical, nonclinical, manufacturing validation or
drug product quality studies
and submit those data before considering or reconsidering the
application. Depending on the extent of these or any other studies,
approval of any applications that we submit may be delayed by
several years, or may require us to expend more resources than we
have available. It is also possible that additional studies, if
performed and completed, may not be considered sufficient by the
FDA or other regulatory agencies.
In addition, the FDA or other regulatory agencies may also approve
a product candidate for fewer
or more limited indications than we request, may impose significant
limitations related to use restrictions for certain age groups,
warnings, precautions or contraindications or may grant approval
contingent on the performance of costly post- marketing clinical
trials or risk mitigation requirements.
We will continue to be subject to
extensive FDA regulation following any product approvals, and if we fail to comply with
these regulations, we may suffer a significant setback in our
business.
Even if we are successful in obtaining regulatory approval of
our product candidates, we will
continue to be subject to the requirements of and review by, the
FDA and comparable regulatory authorities in the areas of
manufacturing processes, post-approval clinical data, adverse event
reporting, labeling, advertising and promotional activities, among
other things. In addition, any marketing approval we receive may be
limited in terms of the approved product indication or require costly
post-marketing testing and surveillance. Discovery after approval
of previously unknown problems with a product, manufacturer or manufacturing process, or
a failure to comply with regulatory requirements, may result in
enforcement actions such as:
●
warning letters or
other actions requiring changes in product manufacturing processes
or restrictions on product marketing or distribution;
●
product recalls or
seizures or the temporary or permanent withdrawal of a product from
the market;
●
suspending any
ongoing clinical trials;
●
temporary or
permanent injunctions against our production
operations;
●
refusal of our
applications for pre-market approval or an investigational new drug
application; and
●
fines, restitution
or disgorgement of profits or revenue, the imposition of civil
penalties or criminal prosecution.
The occurrence of any of these actions would likely cause a
material adverse effect on our business, financial condition and
results of operations.
Many of our business practices are subject to scrutiny and
potential investigation by regulatory and government enforcement
authorities, as well as to lawsuits brought by private citizens
under federal and state laws. We could become subject to
investigations, and our failure to comply with applicable law or an
adverse decision in lawsuits may result in adverse consequences to
us. If we fail to comply with U.S. healthcare laws, we could face
substantial penalties and financial exposure, and our business,
operations and financial condition could be adversely
affected.
While payment is not yet available from third-party payors
(government or commercial) for our products, our goal is to obtain such coverage as
soon as possible after product
approval and commercial launch in the U.S. If this occurs, the availability of such
payment would mean that many healthcare laws would place
limitations and requirements on the manner in which we conduct our
business (including our sales and promotional activities and
interactions with healthcare professionals and facilities) and
could result in liability and exposure to us. In some instances,
our interactions with healthcare professionals and facilities that
occurred prior to commercialization could have implications at a
later date. The laws that may affect our ability to operate
include, among others: (i) the federal healthcare programs
Anti-Kickback Statute, which
prohibits, among other things, persons from knowingly and willfully
soliciting, receiving, offering or paying remuneration, directly or
indirectly, in exchange for or to induce either the referral of an
individual for, or the purchase, order or recommendation of, any
good or service for which payment may be made under federal
healthcare programs such as Medicare or Medicaid, (ii) federal
false claims laws which prohibit, among other things, individuals
or entities from knowingly presenting, or causing to be presented,
claims for payment from Medicare, Medicaid, or other third-party
payors that are false or fraudulent, and which may apply to
entities like us under theories of “implied
certification” where the government and qui tam relators may
allege that device companies
are liable where a product that
was paid for by the government in whole or in part was promoted
“off-label,” lacked necessary approval, or failed to
comply with good manufacturing practices or other laws; (iii)
transparency laws and related reporting and/or disclosures such as
the Sunshine Act; and/or (iv) state law equivalents of each of the
above federal laws, such as anti-kickback and false claims laws
which may apply to items or services reimbursed by any third-party
payor, including commercial insurers, many of which differ from
their federal counterparts in significant ways, thus complicating
compliance efforts.
If our operations are found to be in violation of any of the laws
described above or any other governmental regulations that apply to
us, we may be subject to penalties, including civil and criminal
penalties, exclusion from participation in government healthcare
programs, damages, fines and the curtailment or restructuring of
our operations. Any penalties, damages, fines, curtailment or
restructuring of our operations could adversely affect our ability
to operate our business and our financial results. The risk of our
being found in violation of these laws is increased by the fact
that their provisions are open to a variety of evolving
interpretations and enforcement discretion. Any action against us
for violation of these laws, even if we successfully defend against
it, could cause us to incur significant legal expenses and divert
our management’s attention from the operation of our
business.
Both federal and state government agencies have heightened civil
and criminal enforcement efforts. There are numerous ongoing
investigations of healthcare pharmaceutical companies and others in the healthcare space, as
well as their executives and managers. In addition, amendments to
the Federal False Claims Act, have made it easier for private
parties to bring qui tam (whistleblower) lawsuits against
companies under which the
whistleblower may be entitled to receive a percentage of any money
paid to the government. In addition, the Patient Protection and Affordable Care and Health
Care and Education Affordability Reconciliation Act of 2010
(collectively, the “Affordable Care Act”) amended the
federal civil False Claims Act to provide that a claim that
includes items or services resulting from a violation of the
federal anti-kickback statute constitutes a false or fraudulent
claim for purposes of the federal civil False Claims Act. Penalties
include substantial fines for each false claim, plus three times
the amount of damages that the federal government sustained because
of the act of that person or entity and/or exclusion from the
Medicare program. In addition, a majority of states have adopted
similar state whistleblower and false-claims provision. There can
be no assurance that our activities will not come under the
scrutiny of regulators and other government authorities or that our
practices will not be found to violate applicable laws, rules and
regulations or prompt lawsuits by private citizen
“relators” under federal or state false claims laws.
Any future investigations of our business or executives, or
enforcement action or prosecution, could cause us to incur
substantial costs and result in significant liabilities or
penalties, as well as damage to our reputation.
Laws impacting the U.S. healthcare system are subject to a great
deal of uncertainty, which may result in adverse consequences to
our business.
There have been a number of legislative and regulatory proposals to
change the healthcare system, reduce the costs of healthcare and
change medical reimbursement policies. Doctors, clinics, hospitals
and other users of our products
may decline to purchase our products to the extent there is uncertainty
regarding coverage from government or commercial payors. Further
proposed legislation, regulation and policy changes affecting
third-party reimbursement are likely. Among other things,
Congress has in the past proposed
changes to and the repeal of the Affordable Care Act, and lawsuits
have been brought challenging aspects of the law at various points.
There have been repeated recent attempts by Congress to repeal or replace the Affordable Care
Act. At this time, it remains unclear whether there will be any
changes made to or any repeal or replacement of the Affordable Care
Act, with respect to certain of its provisions or in its entirety.
We are unable to predict what legislation or regulation, if any,
relating to the health care industry or third-party coverage and
reimbursement may be enacted in the future at the state or federal
level, or what effect such legislation or regulation may have on
us. Denial of coverage and reimbursement of our products, or the revocation or changes to coverage
and reimbursement policies, could have a material adverse effect on
our business, results of operations and financial
condition.
We may not be successful in our
efforts to build a pipeline of product candidates.
A key element of our strategy is to use and expand our
product platform to build a pipeline
of product candidates and
progress those product
candidates through clinical development for the treatment of a
variety of different types of cancer. Even if we are successful in
building a product pipeline,
the potential product
candidates that we identify may not be suitable for clinical
development for a number of reasons, including causing harmful side
effects or demonstrating other characteristics that indicate a low
likelihood of receiving marketing approval or achieving market
acceptance. If our methods of identifying potential
product candidates fail to produce a
pipeline of potentially viable product candidates, then our success as a business
will be dependent on the success of fewer potential
product candidates, which introduces
risks to our business model and potential limitations to any
success we may achieve.
Our product candidates may cause undesirable side
effects or have other properties that could delay or prevent their
regulatory approval, limit the commercial profile of an approved
label, or result in significant negative consequences following
marketing approval, if any.
Additionally, if one or more of our
product candidates receives marketing
approval, and we or others later identify undesirable side effects
caused by such products, a
number of potentially significant negative consequences could
result, including:
●
regulatory
authorities may withdraw approvals of such product;
●
regulatory
authorities may require additional warnings on the product’s
label;
●
we may be required
to create a medication guide for distribution to patients that
outlines the risks of such side effects;
●
we could be sued
and held liable for harm caused to patients; and
●
our reputation may
suffer.
Any of these events could prevent us from
achieving or maintaining market acceptance of the particular
product candidate, if approved, and
could significantly harm our business, results of operations and
prospects.
We may expend our limited resources to
pursue a particular product
candidate or indication that does not produce any commercially
viable products and may fail to
capitalize on product
candidates or indications that may be more profitable or for which
there is a greater likelihood of success.
Because we have limited financial and managerial
resources, we must focus our efforts on particular research
programs and product candidates
for specific indications. As a result, we may forego or delay
pursuit of opportunities with other product candidates or for other indications that
later prove to have greater commercial potential. Further, our
resource allocation decisions may result in our use of funds for
research and development programs and product candidates for specific indications that
may not yield any commercially viable products. If we do not accurately evaluate the
commercial potential or target market for a particular
product candidate, we may relinquish
valuable rights to that product
candidate through collaboration, licensing or other royalty
arrangements in cases in which it would have been more advantageous
for us to retain sole development and commercialization rights to
such product candidate. Any
such failure to improperly assess potential product candidates could result in missed
opportunities and/or our focus on product candidates with low market potential,
which would harm our business and financial
condition.
Our products may be expensive to manufacture, and they
may not be profitable if we are unable to control the costs to
manufacture them.
Our products
may be significantly more expensive to manufacture than we expect
or than other therapeutic products currently on the market today. We hope to
substantially reduce manufacturing costs through process
improvements, development of new methods, increases in
manufacturing scale and outsourcing to experienced manufacturers.
If we are not able to make these, or other improvements, and
depending on the pricing of the product, our profit margins may be significantly
less than that of other therapeutic products on the market today. In addition, we may
not be able to charge a high enough price for any
product we develop, even if they are
safe and effective, to make a profit. If we are unable to realize
significant profits from our potential product candidates, our business would be
materially harmed.
We currently lack manufacturing
capabilities to produce our therapeutic product candidates at commercial-scale quantities
and do not have an alternate manufacturing supply, which would
negatively impact our ability to meet any demand for the
product.
We expect that we would need to significantly
expand our manufacturing capabilities to meet potential demand for
our therapeutic product
candidates, if approved. Such expansion would require additional
regulatory approvals. Even if we increase our manufacturing
capabilities, it is possible that we may still lack sufficient
capacity to meet demand.
We do not currently have any alternate supply for
our products. If the facilities
where our products are
currently being manufactured or equipment were significantly
damaged or destroyed, or if there were other disruptions, delays or
difficulties affecting manufacturing capacity or availability of
drug supply, including, but not limited to, if such facilities are
deemed not in compliance with current Good Manufacturing Practice
(“cGMP”) requirements, future clinical studies and
commercial production for our products could be significantly disrupted and
delayed. It would be both time-consuming and expensive to replace
this capacity with third parties, particularly since any new
facility would need to comply with the regulatory
requirements.
Ultimately, if we are unable to supply our
products to meet commercial demand,
whether because of processing constraints or other disruptions,
delays or difficulties that we experience, our production costs
could dramatically increase and sales of our products and their long-term commercial prospects
could be significantly damaged.
To be successful, our proposed
products must be accepted by the
healthcare community, which can be very slow to adopt or
unreceptive to new technologies and products.
Our proposed products and those developed by our collaborative
partners, if approved for marketing, may not achieve market
acceptance since hospitals, physicians, patients or the medical
community in general may decide not to accept and use these
products. The products that we are attempting to develop
represent substantial departures from established treatment methods
and will compete with a number of more conventional therapies
manufactured and marketed by major pharmaceutical
companies. The degree of market
acceptance of any of our developed products will depend on a number of factors,
including:
●
our establishment
and demonstration to the medical community of the clinical efficacy
and safety of our proposed products;
●
our ability to
create products that are superior to alternatives currently on the
market;
●
our ability to
establish in the medical community the potential advantage of our
treatments over alternative treatment methods; and
●
reimbursement
policies of government and third-party payers.
If the healthcare community does not accept our products for any of these reasons, or for any
other reason, our business would be materially
harmed.
Our business is based on novel technologies that are inherently
expensive and risky and may not be understood by or accepted in the
marketplace, which could adversely affect our future
value.
The clinical development, commercialization and
marketing of immuno-oncology therapies are at an early-stage,
substantially research-oriented and financially speculative. To
date, very few companies have
been successful in their efforts to develop and commercialize an
immuno-oncology therapeutic product. In general, such products may be susceptible to various risks,
including undesirable and unintended side effects, unintended
immune system responses, inadequate therapeutic efficacy, or other
characteristics that may prevent or limit their approval or
commercial use. Furthermore, the number of people who may use such
therapies is difficult to forecast with accuracy. Our future
success is dependent on the establishment of a significant market
for such therapies and our ability to capture a share of this
market with our product
candidates.
Our development efforts with our
therapeutic product candidates
are susceptible to the same risks of failure inherent in the
development and commercialization of therapeutic
products based on new technologies.
The novel nature of immuno-oncology therapeutics creates
significant challenges in the areas of product development and optimization,
manufacturing, government regulation, third-party reimbursement and
market acceptance. For example, the FDA has relatively limited
experience regulating such therapies, and there are few approved
treatments using such therapy.
Our competition includes fully
integrated biotechnology and pharmaceutical companies that have significant advantages over
us.
The market for therapeutic immuno-oncology
products is highly competitive. We
expect that our most significant competitors will be fully
integrated and more established pharmaceutical and
biotechnology companies or
institutions, including major multinational pharmaceutical
companies, biotechnology
companies and universities and other
research institutions. These companies are developing similar
products, and they have significantly
greater capital resources and research and development,
manufacturing, testing, regulatory compliance and marketing
capabilities. Many of these potential competitors may be further
along in the process of product
development and also operate large, company-funded research and development programs.
As a result, our competitors may develop more competitive or
affordable products, or achieve
earlier patent protection or product commercialization than we are able to
achieve. Competitive products
may render any products
or product candidates that we
develop obsolete.
Many of our competitors have substantially greater
financial, technical and other resources than we do, such as larger
research and development staff and experienced marketing and
manufacturing organizations. Additional mergers and acquisitions in
the biotechnology and pharmaceutical industries may result in even
more resources being concentrated in certain of our competitors. As
a result, these companies may
be able to obtain regulatory approval more rapidly than we can and
may be more effective in selling and marketing their
products. Smaller or
early-stage companies may also
prove to be significant competitors, particularly through
collaborative arrangements with large, established
companies. Competition may increase
further as a result of advances in the commercial applicability of
technologies and greater availability of capital for investment in
these industries. Our competitors may succeed in developing,
acquiring or licensing drug products that are more effective or less costly to
produce or purchase on the market than any product candidate we are currently developing or
that we may seek to develop in the future. If approved, our
product candidates will face
competition from commercially available drugs as well as drugs that
are in the development pipelines of our
competitors.
Established pharmaceutical companies may invest heavily to accelerate
discovery and development of or in-license novel compounds that
could make our product
candidates less competitive. In addition, any new
product that competes with an
approved product must
demonstrate compelling advantages in efficacy, convenience,
tolerability and safety in order to overcome price competition and
to be commercially successful. Accordingly, our competitors may
succeed in obtaining patent protection, receiving FDA, EMA or other
regulatory approval, or discovering, developing and commercializing
medicines before we do, which could have a material adverse impact
on our business and ability to achieve profitability from future
sales of our approved product
candidates, if any.
If competitors develop and
market products that are more
effective, safer or less expensive than our product candidates or offer other advantages, our
commercial prospects will be limited.
Our therapeutic immuno-oncology development
programs face, and will continue to face, intense competition from
pharmaceutical, biopharmaceutical and biotechnology
companies, as well as numerous
academic and research institutions and governmental agencies
engaged in drug discovery activities or funding, both in the United
States and abroad. Some of these competitors are pursuing the
development of drugs and other therapies that target the same
diseases and conditions that we are targeting with our
product candidates. According to a
recent analysis by InVentiv Health, there are over 800
companies developing approximately
1,500 cancer immunotherapies via 4,000 development projects across
535 targets. According to the Pharmaceutical Manufacturers Research Association
Medicines in Development for Cancer 2018 Report, there were 135
drugs in development for the treatment of lymphoma,
including non-Hodgkin lymphoma,
which accounts for nearly five percent of all new cancer diagnoses.
As a general matter, we also face competition from
many companies that are
researching and developing cell therapies. Many of these
companies have financial and other
resources substantially greater than ours. In addition, many of
these competitors have significantly greater experience in testing
pharmaceutical and other therapeutic products, obtaining FDA and other regulatory
approvals, and marketing and selling. If we ultimately obtain
regulatory approval for any of our product candidates, we also will be competing with
respect to manufacturing efficiency and marketing capabilities,
areas in which we have limited or no commercial-scale experience.
Mergers and acquisitions in the pharmaceutical and biotechnology
industries may result in even more resources’ being
concentrated by our competitors. Competition may increase further
as a result of advances made in the commercial applicability of our
technologies and greater availability of capital for investment in
these fields.
If we are unable to keep up with rapid technological changes in our
field or compete effectively, we will be unable to operate
profitably.
We are engaged in activities in the biotechnology
field, which is characterized by extensive research efforts and
rapid technological progress. If we fail to anticipate or respond
adequately to technological developments, our ability to operate
profitably could suffer. Research and discoveries by other
biotechnology, agricultural, pharmaceutical or other
companies may render our technologies
or potential products or
services uneconomical or result in products superior to those we develop. Similarly,
any technologies, products or
services we develop may not be preferred to any existing or newly
developed technologies, products or services.
We may not be able to obtain
third-party patient reimbursement or favorable product pricing, which would reduce our ability to
operate profitably.
Our ability to successfully commercialize certain
of our proposed products in the
human therapeutic field may depend to a significant degree on
patient reimbursement of the costs of such products and related treatments at acceptable
levels from government authorities, private health insurers and
other organizations, such as health maintenance organizations.
Reimbursement in the United States or foreign countries may not be
available for any products we
may develop, and, if available, may be decreased in the future.
Also, reimbursement amounts may reduce the demand for, or the price
of, our products with a
consequent harm to our business. We cannot predict what additional
regulation or legislation relating to the healthcare industry or
third-party coverage and reimbursement may be enacted in the future
or what effect such regulation or legislation may have on our
business. If additional regulations are overly onerous or
expensive, or if healthcare-related legislation makes our business
more expensive or burdensome than originally anticipated, we may be
forced to significantly downsize our business plans or completely
abandon our business model.
We may be subject to business litigation that will be costly to
defend or pursue and uncertain in its outcome.
Our business may bring us into conflict with our
licensees, licensors or others with whom we have contractual or
other business relationships, or with our competitors or others
whose interests differ from ours. If we are unable to resolve those
conflicts on terms that are satisfactory to all parties, we may become involved in litigation
brought by or against us. That litigation is likely to be expensive
and may require a significant amount of management’s time and
attention, at the expense of other aspects of our business. The
outcome of litigation is always uncertain, and in some cases could
include judgments against us that require us to pay damages, enjoin
us from certain activities, or otherwise affect our legal or
contractual rights, which could have a significant adverse effect
on our business.
We are exposed to the risk of liability claims, for which we may
not have adequate insurance.
Since
we participate in the pharmaceutical industry, we may be subject to
liability claims by employees, customers, end users and third
parties. We intend to obtain proper insurance, however, there can
be no assurance that any liability insurance we purchase will be
adequate to cover claims asserted against us or that we will be
able to maintain such insurance in the future. We intend to adopt
prudent risk-management programs to reduce these risks and
potential liabilities, however, we have not taken any steps to
create these programs and have no estimate as to the cost or time
required to do so and there can be no assurance that such programs,
if and when adopted, will fully protect us. We may not be able to
put risk management programs in place, or obtain insurance, if we
are unable to retain the necessary expertise and/or are
unsuccessful in raising necessary capital in the future. Our
failure to obtain appropriate insurance, or to adopt and implement
effective risk-management programs, as well as any adverse rulings
in any legal matters, proceedings and other matters could have a
material adverse effect on our business.
Preclinical and clinical trials are conducted
during the development of potential products and other treatments to determine their
safety and efficacy for use by humans. Notwithstanding these
efforts, when our treatments are introduced into the marketplace,
unanticipated side effects may become evident. Manufacturing,
marketing, selling and testing our product candidates under development or to be
acquired or licensed, entails a risk of product liability claims. We could be subject
to product liability claims in
the event that our product
candidates, processes, or products under development fail to perform as
intended. Even unsuccessful claims could result in the expenditure
of funds in litigation and the diversion of management time and
resources, and could damage our reputation and impair the
marketability of our product
candidates and processes. While we plan to maintain liability
insurance for product liability
claims, we may not be able to obtain or maintain such insurance at
a commercially reasonable cost. If a successful claim were made
against us, and we lacked insurance or the amount of insurance were
inadequate to cover the costs of defending against or paying such a
claim or the damages payable by us, we would experience a material
adverse effect on our business, financial condition and results of
operations.
We could be subject to
product liability lawsuits based on
the use of our product
candidates in clinical testing or, if obtained, following marketing
approval and commercialization. If product liability lawsuits are brought against us,
we may incur substantial liabilities and may be required to cease
clinical testing or limit commercialization of our
product
candidates.
We could be subject to product liability lawsuits if any
product candidate we develop allegedly
causes injury or is found to be otherwise unsuitable for human use
during product testing,
manufacturing, marketing or sale. Any such product liability claims may include allegations
of defects in manufacturing, defects in design, a failure to warn
of dangers inherent in the product, negligence, strict liability and a breach
of warranties. Claims could also be asserted under state consumer
protection acts. If we cannot successfully defend ourselves
against product liability
claims, we may incur substantial liabilities or be required to
limit commercialization of our product candidates, if approved. Even successful
defense would require significant financial and management
resources. Regardless of the merits or eventual outcome, liability
claims may result in:
●
decreased demand
for our product candidates;
●
withdrawal of
clinical trial participants;
●
initiation of
investigations by regulators;
●
costs to defend the
related litigation;
●
a diversion of
management’s time and our resources;
●
substantial
monetary awards to trial participants or patients;
●
product recalls,
withdrawals or labeling, marketing or promotional
restrictions;
●
loss of revenues
from product sales; and
●
the inability to
commercialize our product candidates.
Our inability to retain sufficient product liability insurance at an acceptable cost
to protect against potential product liability claims could prevent or inhibit
the clinical testing and commercialization of products we develop. We may wish to obtain
additional such insurance covering studies or trials in other
countries should we seek to expand those clinical trials or
commence new clinical trials in other jurisdictions or increase the
number of patients in any clinical trials we may pursue. We also
may determine that additional types and amounts of coverage would
be desirable at later stages of clinical development of our
product candidates or upon commencing
commercialization of any product candidate that obtains required approvals.
However, we may not be able to obtain any such additional insurance
coverage when needed on acceptable terms or at all. If we do not obtain or retain
sufficient product liability
insurance, we could be responsible for some or all of the financial costs associated with
a product liability claim
relating to our preclinical and clinical development activities, in
the event that any such claim results in a court judgment or
settlement in an amount or of a type that is not covered, in whole
or in part, by any insurance policies we may have or that is in
excess of the limits of our insurance coverage. We may not have, or
be able to obtain, sufficient capital to pay any such amounts that
may not be covered by our insurance policies.
We rely on third parties to conduct
preclinical and clinical trials of our product candidates. If these third parties do not
successfully carry out their contractual duties or meet expected
deadlines, we may not be able to obtain regulatory approval for or
commercialize our product
candidates and our business could be substantially
harmed.
We rely, and expect to continue to rely, upon third-party CROs to
execute our preclinical and clinical trials and to monitor and
manage data produced by and relating to those trials. However, we
may not be able to establish arrangements with CROs when needed or
on terms that are acceptable to us, or at all, which could negatively affect our development
efforts with respect to our drug product candidates and materially harm our
business, operations and prospects.
We will have only limited control over the activities of the CRO we
will engage to conduct our clinical trials including the University
of Minnesota for our Phase II
clinical trial for GTB-1550 and Phase I clinical trial for GTB-3550. Nevertheless,
we are responsible for ensuring that each of our studies is
conducted in accordance with the applicable protocol, legal,
regulatory and scientific standards, and our reliance on any CRO
does not relieve us of our regulatory responsibilities. Based on
our present expectations, we, our CROs and our clinical trial sites
are required to comply with good clinical practices
(“GCPs”) for all of
our product candidates in
clinical development. Regulatory authorities enforce GCPs through
periodic inspections of trial sponsors, principal investigators and
trial sites. If we or any of our CROs fail to comply with
applicable GCPs, the clinical data generated in the applicable
trial may be deemed unreliable and the FDA, EMA or comparable
foreign regulatory authorities may require us to perform additional
clinical trials before approving a product candidate for marketing, which we may not
have sufficient cash or other resources to support and which would
delay our ability to generate revenue from any sales of such
product candidate. In addition, our
clinical trials are required to be conducted with
product produced in compliance with
cGMPs. Our or our CROs’ failure to comply with those
regulations may require us to repeat clinical trials, which would
also require significant cash expenditures and delay the regulatory
approval process.
Agreements governing relationships with CROs generally provide
those CROs with certain rights to terminate a clinical trial under
specified circumstances. If a CRO that we have engaged terminates
its relationship with us during the performance of a clinical
trial, we would be forced to seek an engagement with a substitute
CRO, which we may not be able to do on a timely basis or on
commercially reasonable terms, if at all, and the applicable trial would experience
delays or may not be completed. In addition, our CROs are not our
employees, and except for remedies available to us under any
agreements we enter with them, we are
unable to control whether or not they devote sufficient time and
resources to our clinical, nonclinical and preclinical programs. If
CROs do not successfully carry out their contractual duties or
obligations or meet expected deadlines, if they need to be replaced
or if the quality or accuracy of the clinical data they obtain is
compromised due to a failure to adhere to our clinical protocols,
regulatory requirements or for other reasons, our clinical trials
may be extended, delayed or terminated and we may not be able to
obtain regulatory approval for, or successfully commercialize, the
affected product candidates. As
a result, our operations and the commercial prospects for the
effected product candidates
would be harmed, our costs could increase and our ability to
generate revenues could be delayed.
We contract with third parties for the
supply of product candidates
for clinical testing and expect to contract with third parties for
the manufacturing of our product candidates for large-scale testing and
commercial supply. This reliance on third parties increases the
risk that we will not have sufficient quantities of our
product candidates or
products or such quantities at an
acceptable cost, which could delay, prevent or impair our
development or commercialization efforts.
We anticipate continuing our engagement of third parties to provide
our clinical supply as we advance our product candidates into and through clinical
development, and we depend on third parties to produce and maintain
sufficient quantities of material to supply our clinical trials. If
these third parties do not produce and maintain adequate supplies
of clinical material, our development efforts could be
significantly delayed, or could incur substantially higher costs.
We expect in the future to use third parties for the manufacture of
our product candidates for
clinical testing, as well as for commercial manufacture. We plan to
enter into long-term supply agreements with several manufacturers for
commercial supplies. We may be unable to reach agreement on satisfactory terms with contract
manufacturers to manufacture our product candidates. Additionally, the facilities
to manufacture our product
candidates must be the subject of a satisfactory inspection before
the FDA or other regulatory authorities approve a marketing
authorization for the product
candidate manufactured at that facility. We will depend on these
third-party manufacturers for compliance with the FDA’s and
international regulatory authority requirements for the manufacture
of our finished products. We do
not control the manufacturing process of, and are completely
dependent on, our contract manufacturers for compliance with cGMPs.
If our manufacturers cannot successfully manufacture material that
conforms to our specifications and the FDA and other regulatory
authorities’ cGMP requirements, they will not be able to
secure and/or maintain regulatory approval for their manufacturing
facilities. In addition, we have no control over the ability of our
contract manufacturers to maintain adequate quality control,
quality assurance and qualified personnel. If the FDA or a
comparable foreign regulatory authority does not approve these
facilities for the manufacture of our product candidates or if it withdraws any such
approval in the future, we may need to find alternative
manufacturing facilities, which would significantly impact our
ability to develop, obtain regulatory approval for or market
our product candidates, if
approved, and may subject us to recalls or enforcement action
for products already on the
market.
If any of our product candidates are approved and contract
manufacturers fail to deliver the required commercial quantities of
finished product on a timely
basis and at commercially reasonable prices, and we are unable to
find one or more replacement manufacturers capable of production at
a substantially equivalent cost, in substantially equivalent
volumes and quality and on a timely basis, we would likely be
unable to meet demand for our products and could lose potential revenue. It may
take several years to establish an alternative source of supply for
our product candidates and to
have any such new source approved by the FDA or any other relevant
regulatory authorities.
We currently have no marketing and
sales force. If we are unable to establish effective marketing and
sales capabilities or enter into agreements with third parties to market and sell
our product candidates, we may
not be able to effectively market and sell our product candidates, if approved, or
generate product
revenues.
We currently do not have a marketing or sales team for the
marketing, sales and distribution of any of our product candidates that are able to obtain
regulatory approval. In order to commercialize any
product candidates, we must build on a
territory-by-territory basis marketing, sales, distribution,
managerial and other non-technical capabilities or make
arrangements with third parties to perform these services, and we
may not be successful in doing so. If our product candidates receive regulatory approval, we
intend to establish an internal sales and marketing team with
technical expertise and supporting distribution capabilities to
commercialize our product
candidates, which will be expensive and time consuming and will
require significant attention of our executive officers to manage.
Any failure or delay in the development of our internal sales,
marketing and distribution capabilities would adversely impact the
commercialization of any of our products that we obtain approval to market. With
respect to the commercialization of all or certain of our product candidates, we may choose to collaborate,
either globally or on a territory-by-territory basis, with third
parties that have direct sales forces and established distribution
systems, either to augment our own sales force and distribution
systems or in lieu of our own sales force and distribution systems.
If we are unable to enter into such arrangements when needed on
acceptable terms or at all, we
may not be able to successfully commercialize any of our
product candidates that receive
regulatory approval or any such commercialization may experience
delays or limitations. If we are not successful in commercializing
our product candidates, either
on our own or through collaborations with one or more third
parties, our future product
revenue will suffer and we may incur significant additional
losses.
Our business and operations would suffer in the event of system
failures.
Despite the implementation of security measures, our internal
computer systems and those of our contractors and consultants are
vulnerable to damage from computer viruses, unauthorized access,
natural disasters, terrorism, war and telecommunication and
electrical failures. While we have not experienced any such system
failure, accident or security breach to date, if such an event were
to occur and cause interruptions in our operations, it could result
in a material disruption of our drug development programs. For
example, the loss of clinical trial data from completed or ongoing
or planned clinical trials could result in delays in our regulatory
approval efforts and we may incur substantial costs to attempt to
recover or reproduce the data. If any disruption or security breach
resulted in a loss of or damage to our data or applications, or
inappropriate disclosure of confidential or proprietary
information, we could incur liability and/or the further
development of our product
candidates could be delayed.
Our operations are vulnerable to interruption by natural disasters,
power loss, terrorist activity and other events beyond our control,
the occurrence of which could materially harm our
business.
Businesses located in California have, in the past, been subject to
electrical blackouts as a result of a shortage of available
electrical power, and any future blackouts could disrupt our
operations. We are vulnerable to a major earthquake, wildfire and
other natural disasters, and we have not undertaken a systematic
analysis of the potential consequences to our business as a result
of any such natural disaster and do not have an applicable recovery
plan in place. We do not carry any business interruption insurance
that would compensate us for actual losses from interruption of our
business that may occur, and any losses or damages incurred by us
could cause our business to materially suffer.
Epidemic or pandemic outbreaks such as COVID-19 (coronavirus),
natural disasters, whether or not caused by climate change, unusual
weather conditions, terrorist acts and political events, could
disrupt business and result in halting our clinical trials and
otherwise adversely affect our financial performance.
The occurrence of one or more natural disasters, such as tornadoes,
hurricanes, fires, floods and earthquakes, unusual weather
conditions, epidemic outbreaks, terrorist attacks or disruptive
political events in certain regions where our operations are
located could adversely affect our business. Epidemic or pandemic
outbreaks, such as COVID-19 (coronavirus) could impact our
management and our ability to conduct clinical trials. For example,
we were required to temporarily halt our Phase I clinical trial with GTB-3550 for 30 days
in March 2020 as result of restrictions on hospital operation
implemented in reaction to the coronavirus pandemic. This also may
affect the market conditions that would limit our ability to raise
additional capital. This could have a sustained material adverse
effect on our business, financial condition and results of
operations.
We have not held regular annual meetings in the past, and if we are
required by the Delaware Court of Chancery to hold an annual
meeting pursuant to Section 211(c) of the Delaware General
Corporation Law (the “DGCL”) it could result in the
unanticipated expenditure of funds, time and other Company
resources.
Section 2.2 of our bylaws provides that an annual meeting shall be
held each year on a date and at a time designated by our Board of
Directors (the “Board”), and Section 211(b) of the DGCL
provides for an annual meeting of stockholders to be held for the
election of directors. Section 211(c) of the DGCL provides that if
there is a failure to hold the annual meeting for a period of 13
months after the latest to occur of the organization of the
corporation, its last annual meeting or last action by written
consent to elect directors in lieu of an annual meeting, the
Delaware Court of Chancery may order a meeting to be held upon the
application of any stockholder or director. Section 211(c) also
provides that the failure to hold an annual meeting shall not
affect otherwise valid corporate acts or result in a forfeiture or
dissolution of the corporation.
We have not held regular annual meetings in the past because a
substantial majority of our stock is owned by a small number of
stockholders, making it easy to obtain written consent in lieu of a
meeting when necessary. In light of our historical liquidity
constraints, handling matters by written consent has allowed our
Company to save on the financial and administrative resources
required to prepare for and hold such annual meetings. To our
knowledge, no stockholder or director has requested our
Company’s management to hold such an annual meeting and no
stockholder or director has applied to the Delaware Court of
Chancery seeking an order directing our company to hold a meeting. However, if one or more
stockholders or directors were to apply to the Delaware Court of
Chancery seeking such an order, and if the Delaware Court of
Chancery were to order an annual meeting before we are prepared to
hold one, the preparation for the annual meeting and the meeting
itself could result in the unanticipated expenditure of funds, time
and other Company resources.
Risks Related to this Offering and Our Common Stock
There has been a limited public market
for our common stock, and we do
not know whether one will develop to provide you adequate
liquidity. Furthermore, the trading price for our
common stock, should an active trading
market develop, may be volatile and could be subject to wide
fluctuations in per-share price.
Our common stock is quoted on
the OTCQB under the trading symbol “GTBP”;
historically, however, there has been a limited public market for
our common stock. We cannot
assure you that an active trading market for our
common stock will develop or be
sustained. The liquidity of any market for the shares of our
common stock will depend on a number
of factors, including:
●
the number of
stockholders;
●
our operating
performance and financial condition;
●
the market for
similar securities;
●
the extent of
coverage of us by securities or industry analysts; and
●
the interest of
securities dealers in making a market in the shares of our common
stock.
Even if an active trading market develops, the market price for
our common stock may be highly
volatile and could be subject to wide fluctuations. In addition,
the price of shares of our common stock could decline significantly if our
future operating results fail to meet or exceed the expectations of
market analysts and investors and actual or anticipated variations
in our quarterly operating results could negatively affect our
share price.
The volatility of the price of our common stock may also be impacted by the risks
discussed under this “Risk Factors” section, in
addition to other factors, including:
●
developments in the
financial markets and worldwide or regional economies;
●
announcements of
innovations or new products or services by us or our
competitors;
●
announcements by
the government relating to regulations that govern our
industry;
●
significant sales
of our common stock or other securities in the open
market;
●
variations in
interest rates;
●
changes in the
market valuations of other comparable companies; and
●
changes in
accounting principles.
Our outstanding warrants and preferred stock may affect the market
price and liquidity of the common stock.
As of the date of this prospectus, after giving effect to the
issuance of the Settlement Shares and the Settlement Warrants
pursuant to the Settlement Agreement (but excluding the impact of
any conversions of our convertible notes after the filing date of
the registration statement of which this prospectus forms a part),
we had approximately 74.2 million shares of common stock outstanding and had
outstanding warrants for the
purchase of up to approximately 6.8 million additional shares
of common stock at an exercise
price of $0.20 per share, all
of which are exercisable as of the date of this prospectus (subject
to certain beneficial ownership limitations). We also had
outstanding 96,230 shares of Series C preferred stock (the “Series C
Preferred Stock”) and 2,353,548 shares of Series J-1
Preferred Stock as of the date of this prospectus, which preferred
stock is convertible into up to approximately 11.8 million
additional shares of common
stock at any time (subject to certain beneficial ownership
limitations). In addition, as described more fully below, holders
of our convertible notes
and debentures may elect to
receive a substantial number of shares of common stock upon conversion of the
notes and, at each holder’s
option, we will pay accrued interest on such notes in shares of our common stock. The amount of common stock reserved for issuance may have an
adverse impact on our ability to raise capital and may affect the
price and liquidity of our common stock in the public market. In addition,
the issuance of these shares of common stock will have a dilutive effect on
current stockholders’ ownership.
The conversion of outstanding
convertible notes and
debentures into shares of
common stock, and the issuance
of common stock by us as
payment of accrued interest upon our convertible
notes and debentures, could materially dilute our current
stockholders.
As of the date of this prospectus, after giving effect to (i) the
issuance of the July 2020 Notes and the May 2020 Notes and (ii) the
issuance of the Settlement Notes pursuant to the Settlement
Agreement (but excluding the impact of any conversions of our
convertible notes after the filing date of the registration
statement of which this prospectus forms a part), we had
approximately $18.8 million aggregate principal amount of
convertible notes and
debentures outstanding. The
convertible notes and
debentures are convertible into shares
of our common stock at
fixed conversion prices, which
may be less than the market price of our common stock at the time of conversion, and which
may be subject to future adjustment due to certain events,
including the issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
If the entire principal is converted into shares of
common stock (including approximately
$3.9 million in default amounts accrued with respect to the Default
Notes), we would be required to issue an aggregate of no less than
114 million shares of common
stock. If we issue all of these
shares, the ownership of our current stockholders will be
diluted.
Further, at each holder’s option, we will pay interest on the
convertible notes and
debentures in shares of
common stock based on the then
current conversion price. To
date, we have issued approximately 16.7 million shares of
common stock as in-kind interest
payments on our convertible notes and debentures. Such interest payments could further
dilute our current stockholders.
Because our common stock may be deemed a low-priced
“penny” stock, an
investment in our common stock
should be considered high-risk and subject to marketability
restrictions.
Historically, the trading price of our common stock has been $5.00 per share or lower,
and deemed a penny stock, as defined in Rule 3a51-1 under the
Exchange Act, and subject to the penny stock rules of the Exchange
Act specified in rules 15g-1 through 15g-100. Those rules require
broker–dealers, before effecting transactions in any penny
stock, to:
●
deliver to the
customer, and obtain a written receipt for, a disclosure
document;
●
disclose certain
price information about the stock;
●
disclose the amount
of compensation received by the broker-dealer or any associated
person of the broker-dealer;
●
send monthly
statements to customers with market and price information about the
penny stock; and
●
in some
circumstances, approve the purchaser’s account under certain
standards and deliver written statements to the customer with
information specified in the rules.
Consequently, the penny stock rules may restrict the ability or
willingness of broker-dealers to sell the common stock and may affect the ability of holders
to sell their common stock in
the secondary market and the price at which such holders can sell
any such securities. These additional procedures could also limit
our ability to raise additional capital in the
future.
Financial Industry Regulatory
Authority (“FINRA”) sales practice requirements may
also limit a stockholder’s ability to buy and sell our
common stock, which could depress the
price of our common
stock.
In addition to the “penny stock” rules described above,
FINRA has adopted rules that require a broker-dealer to have
reasonable grounds for believing that the investment is suitable
for that customer before recommending an investment to a customer.
Prior to recommending speculative low-priced securities to their
non-institutional customers, broker-dealers must make reasonable
efforts to obtain information about the customer’s financial
status, tax status, investment objectives and other information.
Under interpretations of these rules, FINRA believes that there is
a high probability that speculative low-priced securities will not
be suitable for at least some customers. Thus, the FINRA
requirements make it more difficult for broker-dealers to recommend
that their customers buy our common stock, which may limit your ability to buy
and sell our shares of common
stock, have an adverse effect on the market for our shares
of common stock, and thereby
depress our price per share of common stock.
If securities or industry analysts do not publish research or
reports about our business, or if they issue an adverse or
misleading opinion regarding our stock, our stock price and trading
volume could decline.
The trading market for our common stock may be influenced by the research and
reports that industry or securities analysts publish about us or
our business. We do not currently have, and may never obtain,
research coverage by securities and industry analysts. If no or few
securities or industry analysts commence coverage of us, the
trading price for our common
stock may be negatively affected. In the event that we receive
securities or industry analyst coverage, if any of the analysts who
cover us issue an adverse or misleading opinion regarding us, our
business model, our intellectual property or our stock performance,
or if our operating results fail to meet the expectations of
analysts, our stock price would likely decline. If one or more of
these analysts cease coverage of us or fail to publish reports on
us regularly, we could lose visibility in the financial markets,
which in turn could cause our stock price or trading volume to
decline.
Anti-takeover provisions may limit the ability of another party to
acquire us, which could cause our stock price to
decline.
Delaware law and our restated certificate of incorporation
(“certificate of
incorporation”), our restated bylaws
(“bylaws”) and
other governing documents contain provisions that could discourage,
delay or prevent a third party from acquiring us, even if doing so
may be beneficial to our stockholders, which could cause our stock
price to decline. In addition, these provisions could limit the
price investors would be willing to pay in the future for shares of
our common
stock.
We do not currently or for the
foreseeable future intend to pay dividends on our
common stock.
We have never declared or paid any cash dividends on our
common stock. We currently anticipate
that we will retain future earnings for the development, operation
and expansion of our business and do not anticipate declaring or
paying any cash dividends for the foreseeable future. As a result,
any return on your investment in our common stock will be limited to the appreciation
in the price of our common
stock, if any.
All of the Registered Shares sold pursuant to this prospectus will
be offered and sold by the Selling Stockholders. We will not
receive any proceeds from such sales. We, and not the Selling
Stockholders, will pay all
expenses incident to the registration of the Registered Shares
under the Securities Act.
Our common stock is quoted on
the OTCQB under the symbol “GTBP.” Our
common stock is also quoted on several
European based exchanges, including Berlin (GTBP.BE), Frankfurt (GTBP.DE), the Euronext
(GTBP.NX) and Paris
(GTBP.PA).
Stockholders
As of July 13, 2020, there were 33 stockholders of record, which
total does not include stockholders who hold their shares in
“street name.” The transfer agent for our
common stock is Computershare, whose address is 8742
Lucent Blvd., Suite 225, Highland Ranch, CO
80129.
Dividends
We have not paid any dividends on our common stock to date and do not anticipate that we
will pay dividends in the foreseeable future. Any payment of cash
dividends on our common stock
in the future will be dependent upon the amount of funds legally
available, our earnings, if any, our financial condition, our
anticipated capital requirements and other factors that the Board
may think are relevant. However, we currently intend for the
foreseeable future to follow a policy of retaining
all of our earnings, if any, to
finance the development and expansion of our business and,
therefore, do not expect to pay any dividends on our
common stock during such
time.
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
Overview
We are a clinical stage biopharmaceutical company focused on the
development and commercialization of novel immuno-oncology
products
based off our proprietary Tri-specific Killer Engager (TriKE™) and Tetra-specific Killer Engager (TetraKE™). Our
TriKE and
TetraKE platforms generate proprietary therapeutics designed to
harness and enhance the cancer killing abilities of a
patient’s own NK cells. Once bound to an NK cell, our
moieties are designed to enhance the NK cell and precisely direct
it to one or more specifically-targeted proteins expressed on a
specific type of cancer cell or virus infected cell, ultimately
resulting in the targeted cell’s death. TriKEs and TetraKEs are
made up of recombinant fusion proteins, can be designed to target
any number of tumor antigens on hematologic malignancies, sarcomas
or solid tumors and do not require patient-specific
customization.
Recent Developments
Financings
July 2020 Financing
On July 7, 2020, we entered into a securities purchase agreement with ten purchasers pursuant to
which we issued the July 2020 Notes in an aggregate principal
amount of approximately $3.2 million.
The July 2020 Notes are convertible at any time, at the
holder’s option, into shares of our common stock at an initial conversion price of $0.20 per
share, subject to certain
beneficial ownership limitations (with a maximum ownership limit of
9.99%). The
conversion
price is also subject to adjustment due to certain events,
including stock dividends, stock splits and in connection with the
issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
The July 2020 Notes will be subject to mandatory conversion in the
event of the completion of a future financing in the amount of at
least $15 million at a conversion price equal to the lesser of (i)
the conversion price in effect
for the July 2020 Notes on the date of completion of such financing
or (ii) 75% of the lowest per share price at which
common stock may be issued in
connection with any conversion rights associated with the
financing, in each case, subject to the beneficial ownership
limitations described above.
The July 2020 Notes each have a term of six months and mature on
January 7, 2021, unless earlier converted or repurchased. The July
2020 Notes accrue interest at a rate of 10% per annum, subject to
increase to 18% per annum upon and during the occurrence of an
event of default. Interest is payable in cash or, at the
holder’s option, in shares of common stock based on the conversion price then in effect. We may not prepay
the July 2020 Notes without the prior written consent of the
applicable holder.
May 2020 Financing
Between April 20, 2020 and May 7, 2020, we entered into
securities purchase agreements
with eight purchasers pursuant to which we issued
convertible notes in an
aggregate principal amount of approximately $2.0
million.
The May 2020 Notes are convertible at any time, at the
holder’s option, into shares of our common stock at an initial conversion price of $0.20 per
share, subject to certain
beneficial ownership limitations (with a maximum ownership limit of
9.99%). The
conversion
price is also subject to adjustment due to certain events,
including stock dividends, stock splits and in connection with the
issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
The May 2020 Notes will be subject to mandatory conversion in the
event of the completion of a future financing in the amount of at
least $15 million at a conversion price equal to the lesser of (i)
the conversion price in effect
for the May 2020 Notes on the date of completion of such financing
or (ii) 75% of the lowest per share price at which
common stock may be issued in
connection with any conversion rights associated with the
financing, in each case, subject to the beneficial ownership
limitations described above.
The May 2020 Notes each have a term of six months and mature
between October 20, 2020 and November 7, 2020, unless earlier
converted or repurchased. The May 2020 Notes accrue interest at a
rate of 10% per annum, subject to increase to 18% per annum upon
and during the occurrence of an event of default. Interest is
payable in cash or, at the holder’s option, in shares
of common stock based on
the conversion price then in
effect. We may not prepay the May 2020 Notes without the prior
written consent of the applicable holder.
January 2020 Financing
On January 30, 2020, we entered into a securities
purchase agreement with one purchaser
pursuant to which we issued the January 2020 Notes in an aggregate
principal amount of $0.2 million.
The January 2020 Notes are convertible at any time, at the
holder’s option, into shares of our common stock at an initial conversion price of $0.20 per
share, subject to certain
beneficial ownership limitations (with a maximum ownership limit of
9.99%). The
conversion
price is also subject to adjustment due to certain events,
including stock dividends, stock splits and in connection with the
issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
The January 2020 Notes have a term of eight months and mature on
September 30, 2020, unless earlier converted or repurchased. The
January 2020 Notes accrue interest at a rate of 10% per annum,
subject to increase to 18% per annum upon and during the occurrence
of an event of default. Interest is payable in cash or, at the
holder’s option, in shares of common stock based on the conversion price then in effect. We may not prepay
the January 2020 Notes without the prior written consent of the
holder.
For additional information regarding the terms of our
convertible notes and
debentures, including the Applicable
Notes, and the securities purchase agreements pursuant to which they were
issued, see “Indebtedness—Convertible
Notes/Debentures”
below.
Forbearance Agreements
Effective as of June 23, 2020, we entered into the Forbearance
Agreements with the holders of approximately $13.2 million
aggregate principal amount of the Default Notes, which are
currently in default. Pursuant to the Forbearance Agreements, the
holders of the Default Notes have agreed to forbear from exercising
their rights and remedies under the Default Notes (including
declaring such Default Notes (together with default amounts and
accrued and unpaid interest) immediately due and payable) until the
earlier of (i) the date that we complete a New Financing or (ii)
the Termination Date.
Pursuant to the Forbearance Agreement, the holders of the Default
Notes have also agreed that the Default Notes (together with
default amounts and accrued and unpaid interest) will be converted
into common stock upon the
closing of a New Financing at a conversion price equal
to the lesser of (i) the conversion price in
effect for the Default Notes on the date of such New Financing or
(ii) 75% of the lowest per share price at which common stock is or may
be issued in connection with such New Financing, in each case,
subject to certain beneficial ownership limitations (with a maximum
ownership limit of 9.99%). Shares of our Series
J-1 Preferred Stock, which are convertible into the
Company’s common stock, will be
issued in lieu of common stock to the
extent that conversion of the Default Notes is prohibited by such
beneficial ownership limitations.
For additional information regarding the terms of the Forbearance
Agreements, see “Indebtedness—Forbearance
Agreements” below.
Settlement with Empery Funds
Settlement Agreement
On June 19, 2020, we entered into the Settlement Agreement with the
Empery Funds, Anthony Cataldo and Paul Kessler resolving
all
remaining disputes between the parties pertaining to the
Original
Securities issued by the Company to the Empery Funds in January
2018 pursuant to a securities purchase agreement. See
“Description
of Business—Legal Proceedings.”
As a result of the Settlement Agreement, the Company paid the
Empery Funds cash payments in an aggregate amount of $0.2 million.
In addition, pursuant to the Settlement Agreement, the Company
issued to the Empery Funds, solely in exchange for the
outstanding Original Securities,
(i) an aggregate of 3.5 million shares of common stock, (ii)
pre-funded warrants to purchase an
aggregate of 5.5 million shares of common stock and (iii)
senior convertible notes in an aggregate
principal amount of $0.45 million.
Settlement Notes
The Settlement Notes are convertible
at any time, at the holder’s option, into shares of
common
stock at an initial conversion rate of $0.20 per share, subject to
certain beneficial ownership limitations (with a maximum ownership limit of
4.99%). The
conversion
price is also subject to adjustment due to certain events,
including stock dividends, stock splits and in connection with the
issuance by the Company of common stock or
common
stock equivalents at an effective price per share lower than the
conversion rate then in effect.
The Settlement Notes have a term of
six months, maturing on December 19, 2020, and bear interest at a
rate of 10% per annum, subject to increase to 18% per annum upon
and during the occurrence of an event of default. Interest is
payable in cash or, at the holder’s option, in shares
of common stock based on
the conversion price then
in effect.
Pursuant to the terms of the Settlement Notes, the Company is
required to make an offer to repurchase, at the holder’s
option, the Settlement Notes at price in cash
equal to 100% of the aggregate principal amount of the
Settlement Note plus accrued and
unpaid interest, if any, to, but excluding, the date of repurchase
following the consummation by the Company of a capital raising
transactions, or a series of transactions, resulting in aggregate
gross proceeds to the Company in excess of $7.5 million. The
Company may not otherwise prepay the Settlement Notes without the prior
written consent of the applicable Empery Funds.
For additional information regarding the terms of the Settlement
Notes and Settlement Agreement, see “Indebtedness—Convertible
Notes/Debentures”
below.
Settlement Warrants
The Settlement Warrants provide for the purchase of up to an
aggregate of 5.5 million shares of common stock at an
exercise price of $0.20 per share, subject to adjustment in certain
circumstances, and expire on June 19, 2025. Exercise of the
warrant is
subject to certain additional terms and conditions, including
certain beneficial ownership limitations (with a maximum ownership limit of
4.99%).
Collaboration Agreement
On March 10, 2020, we entered into a collaboration
agreement
with Cytovance®
Biologics, a USA-based contract development and manufacturing
organization and a subsidiary of Hepalink, to provide development
services for a TriKE therapeutic for
the treatment of the coronavirus infection. Under the terms of the
collaboration agreement, the
companies
will focus on preparing sufficient quantities of our
coronavirus TriKE drug
product for
preclinical evaluation using Cytovance’s E.
coli-based Keystone
Expression System™ and
subsequently, will scale-up production using Cytovance’s GMP
microbial manufacturing platform for evaluation of
TriKE in
humans to treat the coronavirus infection.
Results of Operations
Comparison of the Three Months Ended March 31, 2020 and
2019
Research and Development Expenses
During the three months ended March 31, 2020 and 2019, we incurred
$324,000 and $834,000 of research and development expenses,
respectively. Research and development costs decreased due
primarily to reductions in employees, consultants and preclinical
expenses. We anticipate our direct clinical costs to increase in
second half of 2020 upon the continuation of a Phase I clinical trial of our most advanced
TriKe product candidate, GTB-3550.
Selling, general and administrative expenses
During the three months ended March 31, 2020 and 2019, we incurred
$746,000 and $3,222,000 of selling, general and administrative
expenses, respectively. The decrease in selling, general and
administrative expenses is primarily attributable the reduction of
salaries.
Interest Expense
Interest expense was $638,000 and $454,000 for the three months
ended March 31, 2020 and 2019, respectively. The increase is
primarily due to the accrual of interest on outstanding
convertible notes and
debentures.
Comparison of the Fiscal Years Ended December 31, 2019 and
2018
Research and Development Expenses
During the years ended December 31, 2019 and 2018, we incurred $1.7
million and $9.1 million of research and development expenses,
respectively. Research and development costs in 2018 were high due
primarily to the addition of new employees, increased regulatory
and preclinical consultant costs to support the GTB-3550 IND,
higher costs to advance the central nervous system
(“CNS”) portfolio and position the assets for licensing
efforts, and higher preclinical and clinical expenses incurred at
the University of Minnesota to continue development of our
immune-oncology assets. Expenses in 2018 also include non-cash
compensation of $6.8 million. We anticipate our direct clinical and
preclinical costs to continue to increase throughout 2020, totaling
approximately $12 million to $15 million, as we have initiated
the Phase I clinical trial of
our most advanced TriKe
product candidate, GTB-3550 in January
2020, and initiate IND-enabling activities for GTB-C3550 and
GTB-1615 later in 2020.
Selling, general and administrative expenses
During the years ended December 31, 2019 and 2018, we incurred $9.7
million and $12.5 million of selling, general and administrative
expenses, respectively. Additional selling, general, and
administrative expenses in 2018 were due to increased spending on
investor relations campaigns to broaden awareness of the Company
and increased legal costs primarily associated with regulatory and
financing efforts. We anticipate selling, general and
administrative expenses, excluding stock compensation, to range
between $1 million and $2 million in the coming
quarters.
Loss on impairment
For the year ended December 31, 2018, the Company recorded an
intangible asset impairment charge of $228.5 million related to
the CNS In-Process Research & Development
(“IPR&D”) assets, which represents the excess
carrying value compared to fair value. The impairment charge was
the result of both internal and external factors. In the 3rd
quarter of 2018, the Company experienced changes in key senior
management, led by the appointment of a CEO with extensive
experience in oncology drug development. These changes resulted in
the prioritization for immuno-oncology development candidates
relative to the CNS development candidates acquired from Georgetown
Translational Pharmaceuticals. In conjunction with these strategic
changes, limited internal resources have delayed the development of
the CNS IPR&D assets. The limited resources, changes in senior
leadership, and favorable market conditions for immuno-oncology
development candidates have resulted in the Company choosing to
focus on development of its immuno-oncology
portfolio.
On September 19, 2019, the Company entered into an Asset
Purchase Agreement (the
“APA”), pursuant to which the Company sold its rights,
titles and interests, including associated patents, to the
pharmaceutical product designated by the Company as GTB-004 (the “Product”). Under the APA, the Product was
purchased by DAS Therapeutics, Inc. which the Company believes is
well positioned to take over the clinical development of the
Product including obtaining timely approval by the
FDA.
The Company received $200,000 at closing. The Company will also
participate in any future commercial value of the Product by
receiving $6,000,000 upon the achievement of certain sales
objectives. In addition, the Company will receive a royalty equal
to 1.5% of U.S. sales until such time as the last of the patents
associated with the Product expires. The Company reflected a loss
in the year ended December 31, 2019 totaling
$20,463,000.
As a result of the loss reported on the sale of the Product, as
well as the response received on inquiries related to the other two
projects, the Company determined that the remaining value related
to these remaining projects should be fully impaired. During the
year ended December 31, 2019, the Company reported an impairment
charge for these projects totaling $4,599,000.
Interest Expense
Interest expense was $2.1 million and $9.1 million for the years
ended December 31, 2019 and 2018, respectively. The decrease is due
to a decrease in non-cash amortization of debt issuance costs
associated with convertible notes and debentures and warrants issued in January
2018.
Liquidity and Capital Resources
The Company’s current operations have focused on business
planning, raising capital, establishing an intellectual property
portfolio, hiring and conducting preclinical studies and clinical
trials. The Company does not have any product candidates approved for sale and has not
generated any revenue from product sales. The Company has sustained operating
losses since inception and expects such losses to continue over the
foreseeable future. During the three months ended March 31, 2020,
the Company raised $200,000 through the issuance of the January
2020 Notes. In the second quarter and third quarter of 2020, the
Company has also issued an additional approximately $5.7 million
aggregate principal amount of the May 2020 Notes and the July 2020
Notes. We anticipate that cash
utilized for selling, general and administrative expenses will
range between $1 and $2 million in the coming quarters, while
research and development expenses will vary depending on clinical
activities. The Company is pursuing several alternatives to address
this situation, including the raising of additional funding through
equity or debt financings. In order to finance existing operations
and pay current liabilities over the next 12 months, the Company
will need to raise an additional $15 million of capital in
2020.
The financial statements of the Company have been prepared on a
going-concern basis, which contemplates the realization of assets
and the satisfaction of liabilities in the normal course of
business. Accordingly, the financial statements do not include any
adjustments that might be necessary should the Company be unable to
continue in existence.
The Company has incurred substantial losses and negative cash flows
from operations since its inception and has an accumulated deficit
of $569 million and cash of $5 thousand as of March 31, 2020. The
Company anticipates incurring additional losses until such time, if
ever, that it can generate significant sales or revenue from
out-licensing of its products
currently in development. Substantial additional financing will be
needed by the Company to fund its operations and to commercially
develop its product candidates.
These factors raise substantial doubt about the Company’s
ability to continue as a going concern.
Management is currently evaluating different strategies to obtain
the required funding for future operations. These strategies may
include but are not limited to: public offerings of equity and/or
debt securities, payments from potential strategic research and
development, licensing and/or marketing arrangements with
pharmaceutical companies.
Management has also implemented cost saving efforts, including
reduction in executive salaries and reduced travel. Management
believes that these ongoing and planned financing endeavors, if
successful, will provide adequate financial resources to continue
as a going concern for at least the next six months from the date
the financial statements are issued; however, there can be no
assurance in this regard. If the Company is unable to secure
adequate additional funding, its business, operating results,
financial condition and cash flows may be materially and adversely
affected.
Indebtedness
Convertible Notes/Debentures
As of the date of this prospectus, after giving effect to (i) the
issuance of the July 2020 Notes and the May 2020 Notes and (ii) the
issuance of the Settlement Notes pursuant to the Settlement
Agreement (but excluding the impact of any conversions of our
convertible notes after the filing date of the registration
statement of which this prospectus forms a part), we had
approximately $18.8 million aggregate principal amount of
convertible notes and
debentures outstanding that were
issued pursuant to securities purchase agreements (or, in the case of the
Settlement Notes, the
Settlement Agreement) entered into with numerous
investors.
The convertible notes
and debentures are convertible
at any time, at the holder’s option, into shares of
our common stock at an initial
conversion rate, subject to certain beneficial ownership
limitations (which vary between maximum ownership of between 4.99%
and 9.99%). The conversion
price is also generally subject to adjustment due to certain
events, including stock dividends, stock splits and in connection
with the issuance by the Company of common stock or common stock equivalents at an effective price per
share lower than the conversion rate then in effect. The
conversion price for each of our
outstanding convertible notes
and debentures is currently
$0.20 per share. In addition, the July 2020 Notes and the May 2020
Notes will be subject to mandatory conversion in connection with
the completion of a future financing in the amount of at least $15
million, subject to the beneficial ownership limitations described
above.
The convertible notes
and debentures generally have
terms of six months to one year. The convertible
notes and debentures each accrue interest at a rate of 10%
per annum, subject to increase to 18% per annum upon and during the
occurrence of an event of default with respect to certain of our
convertible notes and
debentures. Interest is payable in
cash or, with respect to certain of our convertible
notes and debentures, and at the holder’s option, in
shares of common stock based on
the conversion price then in
effect.
Pursuant to the terms of the Settlement Notes, the Company is required to make an offer to
repurchase, at the holder’s option, the Settlement
Notes at price in cash equal to 100%
of the aggregate principal amount of the Settlement
Note plus accrued and unpaid interest,
if any, to, but excluding, the date of repurchase following the
consummation by the Company of a capital raising transactions, or a
series of transactions, resulting in aggregate gross proceeds to
the Company in excess of $7.5 million. Generally, we otherwise do
not have the right to prepay any of the convertible
notes and debentures without the prior written consent of
the holders of such securities.
The convertible notes
and debentures contain a number
of affirmative and negative covenants and customary events of
default. See “Risk Factors—Risks
Related to Our Business—Our current and future indebtedness
may impose significant operating and financial restrictions on us
and affect our ability to access liquidity.”
The securities purchase
agreements and Settlement Agreement, as applicable, also generally
contain certain ongoing covenants of the Company, including rights
of participation in certain future financing transactions,
limitations on future variable
rate transactions at “at-the-market” offerings and
“most favored nation” provisions giving holders of
certain of the convertible notes and debentures the benefit of any terms or conditions
under which the Company agrees to issue or sell any
common stock or common stock equivalents that are more favorable
to an investor than the terms and conditions granted to such holder
under the applicable securities purchase agreement and the transactions
contemplated thereby.
The convertible notes
and debentures are senior
obligations of the Company. In addition, approximately $1.4 million
aggregate principal amount of the convertible note and debenture are secured by a first priority security
interest in substantially all
of the assets of the Company and its subsidiaries. Certain
convertible note and
debentures are also secured by
individual pledges by certain of our current and former officers
and directors of our common
stock owned by such officer and directors.
For additional information about our convertible
notes and debentures, see Note 2 to our unaudited financial
statements, Debt.
Forbearance Agreements
Effective as of June 23, 2020, we entered into the Forbearance
Agreements with the holders of $13.2 million aggregate principal
amount of the Default Notes, which are currently in default.
Pursuant to the Forbearance Agreements, the holders of the Default
Notes have agreed to forbear from exercising their rights and
remedies under the Default Notes (including declaring such Default
Notes (together with default amounts and accrued and unpaid
interest) immediately due and payable) until the earlier of (i) the
date that we complete a New Financing or (ii) the Termination Date.
As a result of the ongoing default, the Default Notes are currently
accruing interest at the default rate of 18% per annum and have
also accrued defaults in an aggregate amount of $3.9
million.
The obligations of the holders to forbear from exercising their
rights and remedies under the Default Notes pursuant to the
Forbearance Agreements will terminate on the earliest of (i) the
Termination Date, (ii) the date of any bankruptcy filing by the
Company or its subsidiaries, (iii) the date on which the Company
defaults on any of the terms and conditions of the Forbearance
Agreements or (iv) the date the Forbearance Agreements are
otherwise terminated or expire.
The Forbearance Agreements contain various customary and other
representations, warranties and covenants of the Company and the
holders of the Default Notes, including an agreement that the
Default Notes (together with default amounts and accrued and unpaid
interest) will be converted into common stock upon the
closing of a New Financing at a conversion price equal
to the lesser of (i) the conversion price in
effect for the Default Notes on the date of such New Financing or
(ii) 75% of the lowest per share price at which common stock is or may
be issued in connection with such New Financing, in each case,
subject to certain beneficial ownership limitations (with a maximum
ownership limit of 9.99%). Shares of our Series
J-1 Preferred Stock, which are convertible into the
Company’s common stock, will be
issued in lieu of common stock to the
extent that conversion of the Default Notes is prohibited by such
beneficial ownership limitations.
Gemini Financing Agreement
On November 8, 2010, the Company entered into a financing
arrangement with Gemini Pharmaceuticals, Inc., a
product development and manufacturing
partner of the Company, pursuant to which Gemini Pharmaceuticals
made a $250,000 strategic equity investment in the Company and
agreed to make a $750,000 purchase order line of credit facility
available to the Company. The outstanding principal of
all advances under the line of credit
bear interest at the rate of interest of prime plus 2% per annum.
There is $31,000 due on this credit line at March 31,
2020.
Critical Accounting Policies
We consider the following accounting policies to be critical given
they involve estimates and judgments made by management and are
important for our investors’ understanding of our operating
results and financial condition.
Basis of Consolidation
The consolidated financial statements contained in this report
include the accounts of GT Biopharma, Inc. and its
subsidiaries. All intercompany
balances and transactions have been eliminated.
Long-Lived Assets
Our long-lived assets include property, plant and equipment,
capitalized costs of filing patent applications and goodwill and
other assets. We evaluate our long-lived assets for impairment in
accordance with Accounting
Standards Codification (“ASC”) 360, whenever events or changes in
circumstances indicate that the carrying amount of such assets may
not be recoverable. Estimates of future cash flows and timing of
events for evaluating long-lived assets for impairment are based
upon management’s judgment. If any of our intangible or
long-lived assets are considered to be impaired, the amount of
impairment to be recognized is the excess of the carrying amount of
the assets over its fair value.
Applicable long-lived assets are amortized or depreciated over the
shorter of their estimated useful lives, the estimated period that
the assets will generate revenue, or the statutory or contractual
term in the case of patents. Estimates of useful lives and periods
of expected revenue generation are reviewed periodically for
appropriateness and are based upon management’s judgment.
Goodwill and other assets are not amortized.
Certain Expenses and Liabilities
On an ongoing basis, management evaluates its estimates related to
certain expenses and accrued liabilities. We base our estimates on
historical experience and on various other assumptions that we
believe to be reasonable under the circumstances, the results of
which form the basis for making judgments about the carrying values
of liabilities that are not readily apparent from other sources.
Actual results may differ materially from these estimates under
different assumptions or conditions.
Inflation
We believe that inflation has not had a material adverse impact on
our business or operating results during the periods
presented.
Off-balance Sheet Arrangements
We have no off-balance sheet arrangements as of March 31,
2020.
DESCRIPTION OF BUSINESS
We are a clinical stage biopharmaceutical company focused on the development and
commercialization of novel immuno-oncology products based off our proprietary
Tri-specific Killer Engager
(TriKE™) and
Tetra-specific Killer Engager
(TetraKE™). Our
TriKE and TetraKE platforms generate
proprietary therapeutics designed to harness and enhance the cancer
killing abilities of a patient’s own NK cells. Once bound to
an NK cell, our moieties are designed to enhance the NK cell and
precisely direct it to one or more specifically-targeted proteins
expressed on a specific type of cancer cell or virus infected cell,
ultimately resulting in the targeted cell’s death.
TriKEs and TetraKEs are made up of
recombinant fusion proteins, can be designed to target any number
of tumor antigens on hematologic malignancies, sarcomas or solid
tumors and do not require patient-specific
customization.
We are using our TriKE and
TetraKE platforms with the intent to bring to market
immuno-oncology products that
can treat a range of hematologic malignancies, sarcoma and solid
tumors. The platforms are scalable, and we are putting processes in
place to be able to produce IND-ready moieties in a timely manner
after a specific TriKE or
TetraKE conceptual design. After conducting market and competitive
research, specific moieties can then be advanced into the clinic on
our own or through potential collaborations with larger
companies. We are also evaluating, in
conjunction with our Scientific
Advisory Board, additional moieties designed to target different
tumor antigens. We believe our TriKEs and TetraKEs may have the ability, if
approved for marketing, to be used on a stand-alone basis, augment
the current monoclonal antibody therapeutics, be used in
conjunction with more traditional cancer therapy and potentially
overcome certain limitations of current CAR-T
therapy.
We are also using our TriKE and
TetraKE platforms to develop therapeutics useful for the treatment
of infectious disease such as for the treatment of patients
infected by HIV. While the use of anti-retroviral drugs has
substantially improved the health and increased the longevity of
individuals infected with HIV, these drugs are designed to suppress
virus replication to help modulate progression to AIDS and to limit
further transmission of the virus. Despite the use of
anti-retroviral drugs, infected individuals retain reservoirs of
latent HIV-infected cells that, upon cessation of anti-retroviral
drug therapy, can reactivate and reestablish an active HIV
infection. For a curative therapy, destruction of these latent HIV
infected cells must take place. The HIV-TriKE contains the antigen binding fragment
(Fab) from a
broadly-neutralizing antibody targeting the HIV-Env protein. The HIV-TriKE is designed to target HIV while
redirecting NK cell killing specifically to actively replicating
HIV infected cells. The HIV-TriKE induced NK cell proliferation and
demonstrated the ability in vitro to reactivate and kill
HIV-infected T-cells. These findings indicate a potential role for
the HIV-TriKE in the
reactivation and elimination of the latently infected HIV reservoir
cells by harnessing the NK cell’s ability to mediate the
ADCC.
Our initial work has been conducted in collaboration with
the Masonic Cancer Center at
the University of Minnesota under a program led by Dr. Jeffrey
Miller, the Deputy Director.
Dr. Miller is a recognized leader in the field of NK cell and IL-15
biology and their therapeutic potential. We have exclusive rights
to the TriKE and TetraKE
platforms and are generating additional intellectual property
around specific moieties.
Immuno-Oncology Platform
Tri-specific Killer Engagers
(TriKEs) and
Tetra-specific Killer Engagers
(TetraKEs)
The generation of chimeric antigen receptor (“CAR”)
expressing T cells from monoclonal antibodies has represented an
important step forward in cancer therapy. These therapies involve
the genetic engineering of T cells to express either CARs, or T
cell receptors, (“TCRs”), and are designed such that the
modified T cells can recognize and destroy cancer cells. While a
great deal of interest has recently been placed upon CAR-T therapy,
it has certain limitations for broad potential applicability
because it can require an individual approach that is expensive and
time consuming, and may be difficult to apply on a large scale. NK
cells represent an important immunotherapeutic target as they are
involved in tumor immune-surveillance, can mediate ADCC, contain
pre-made granules with perforin and granzyme B and can quickly
secrete inflammatory cytokines, and unlike T cells they do not
require antigen priming and can kill cells in the absence of major
histocompatibility complex (“MHC”) presentation of
antigens. We believe there is a continued unmet medical need for
targeted immuno-oncology therapies that can have the potential to
be dosed in a patient-friendly outpatient setting, can be used on a
stand-alone basis, augment the current monoclonal antibody
therapeutics or be used in conjunction with more traditional cancer
therapy. We believe our TriKE
and TetraKE constructs have this potential and therefore we have
generated, and intend to continue to generate, a pipeline of
product candidates to be advanced into
the clinic on our own or through potential collaborations with
larger companies.
GTB-3550 TriKE™ and GTB-3550 TriKE™ Phase I/II Clinical
Trial
GTB-3550 is the Company’s first TriKE™ product candidate which is a single-chain,
tri-specific recombinant fusion protein construct composed of the
variable regions of the heavy and light chains of anti-CD16 and
anti-CD33 antibodies and a modified form of IL-15. The
GTB-3550 Phase I/II clinical
trial for treatment of patients with CD33-expressing, high
risk MDSs, refractory/relapsed
acute myeloid leukemia or advanced systemic mastocytosis opened for
patient enrollment September 2019. The clinical trial is being
conducted at the University of
Minnesota’s Masonic Cancer Center in Minneapolis, Minnesota
under the direction of Dr. Erica Warlick.
NK cells represent an important immunotherapeutic target as they
are involved in tumor immune-surveillance, can mediate ADCC,
contain pre-made granules with perforin and granzyme B and can
quickly secrete inflammatory cytokines, and unlike T cells they do
not require antigen priming and can kill cells in the absence of
MHC presentation.
Unlike full-length antibodies, TriKEs and TetraKEs are small single-chain fusion
proteins that bind the CD16 receptor of NK cells directly producing
a potent and lasting response, as demonstrated by preclinical
studies. An additional benefit they may have is attractive
biodistribution, as a consequence of their smaller size, which we
expect to be important in the treatment of solid tumors. In
addition to these advantages, TriKEs and TetraKEs are designed to be
non-immunogenic, have appropriate clearance properties and can be
engineered quickly to target a variety of tumor
antigens.
Background and Select Non-Clinical Data
In conjunction with our research agreement with the Masonic Cancer Center at the University of
Minnesota, the exploration of targeting NK cells to a variety of
tumors initially focused on novel bi-specific killer engagers
(“BiKEs”) composed of the variable portions of
antibodies targeting the CD16 activating receptor on NK cells and
CD33 (AML and MDS; see figure below), CD19/CD22 (B cell lymphomas),
or EpCAM (epithelial tumors
(breast, colon and lung)) on the tumor cells.
Subsequently, a tri-specific (TriKE) construct that replaced the linker molecule
between the CD16
scFv and the CD33 scFv with
a modified IL-15 molecule, containing flanking sequences, was
generated and tested. Data indicate that the CD16 x IL-15 x CD33
and CD16 x IL-15 x EpCAM TriKEs
potently induce proliferation of healthy donor NK cells, possibly
greater than that induced by exogenous IL-15, which is absent in
the BiKE platform. Targeted delivery of the IL-15 through
the TriKE also resulted in
specific expansion of the NK cells without inducing T cell
expansion on post-transplant patient samples.
When compared to the CD16 x CD33 BiKE, the CD16 x IL-15 x CD33 TriKE is also capable of potently restoring
killing capacity of post-transplant NK cells against
CD33-expressing HL-60 targets
and primary AML blasts. These results demonstrated the ability to
functionally incorporate an IL-5 cytokine into the BiKE platform
and also demonstrated the possibility of targeting a variety of
cytokines directly to NK cells while reducing off-target effects
and the amount of cytokines needed to obtain biologically relevant
function.
The figure below is a schematic of a BiKE construct (top) and
a TriKE construct (bottom),
which has the modified IL-15 linker between the CD16 scFv and
the CD33 scFv components.
The TriKE constructs were also
tested against three separate human tumor cell lines: HL-60
(promyelocitic leukemia), Raji
(Burkitt’s lymphoma) and
HT29 (colorectal adenocarcinoma), in addition to a model for
ovarian cancer. All cell lines
contained the Luc reporter to
allow for in vivo imaging of the tumors. These systems were used to
show in vivo efficacy of BiKEs (1633) and TriKEs (GTB-3550) against relevant human tumor
targets (HL-60-luc) over an extended period of time. The system
consisted of initial conditioning of mice using radiation
(250-275 cGy), followed by
injection of the tumor cells (I.V. for HL-60-luc and
Raji-luc, intra-splenic for HT29-luc
and IP for ovarian for MA-148-luc), a three-day growth phase,
injection of human NK cells and repeated injection of the drugs of
interest, BiKE and TriKE (three
to five times a week). Imaging was carried out at day 7, 14 and 21,
and extended as needed.
Figure A below shows the results (tumor burden and mortality) when
dosing NK cells alone (top panel), the BiKE version (lacking IL-15)
of GTB-3550 (middle panel; called 1633) and the TriKE, GTB-3550 (bottom panel; then called 161533)
in the above described human tumor model, HL-60-luc. In the
NK-cell-only arm, two out of the five mice were dead by day 21 with
two of the surviving mice having extensive tumor burden as depicted
by the colored images. In contrast, all five mice in each of the BiKE and TriKE arms survived. In addition, the
tumor burden in the TriKE-treated mice was significantly less than in
the BiKE-treated mice,
demonstrating the improved efficacy from NK cells in the
TriKE-treated
mice.
Based on these results, and others, the IND for GTB-3550 was filed
in June 2017 by the University of Minnesota. The FDA requested that
additional preclinical toxicology be conducted prior to initiating
clinical trials. The FDA also requested some additional information
and clarifications on the manufacturing and clinical packages. The
requested additional information and clarifications were completed
and incorporated by us into the IND in eCTD format. We filed the IND amendment in
June 2018 and announced on November 1, 2018 that we had received
notification from the FDA that the IND was open and the Company was
authorized to initiate a first-in-human Phase I study with GTB-3550 in AML, MDS and severe
mastocytosis. We began the Phase I clinical trial in January
2020.
Generation of humanized single-domain
antibody targeting CD16 for incorporation into the
TriKE platform
To develop second generation TriKEs, we designed a new humanized CD16 engager
derived from a single-domain antibody. While scFvs consist of a heavy and a light variable
chain joined by a linker, single-domain antibodies consist of a
single variable heavy chain capable of engaging without the need of
a light chain counterpart (see figure below).
These single-domain antibodies are thought to have certain
attractive features for antibody engineering, including physical
stability, ability to bind deep grooves and increased production
yields, amongst others. Pre-clinical studies demonstrated increased
activity (NK Cell
Degranulation) and functionality (NC Cell Cytokine Production) of the
single-domain CD16 TriKE
(GTB-C3550) compared to the original TriKE (GTB-3550) (see figure below). This data was
presented at the 2017 American
Society of Hematology Conference.
Targeting Solid Tumors and Other Potentially Attractive
Characteristics
Unlike full-length antibodies, TriKEs and TetraKEs are small single-chain fusion
proteins that bind the CD16 receptor of NK cells directly producing
a potentially more potent and lasting response as demonstrated by
preclinical studies. An additional benefit that they may have is an
attractive biodistribution, because of their smaller size, which we
expect to be important in the treatment of solid tumors. In
addition to these potential advantages, TriKEs and TetraKEs are designed to be
non-immunogenic, have appropriate clearance properties and can be
engineered quickly to target a variety of tumor antigens. We
believe these attributes make them an ideal pharmaceutical platform
for potentiated NK cell-based immunotherapies and have the
potential to overcome some of the limitations of CAR-T therapy and
other antibody therapies.
Examples of our earlier stage solid tumor targeting
product candidates are focused
on EpCAM, Her2, Mesothelin (mesothelioma and lung adenocarcinoma)
and CD133 alone and in combination. We believe certain of these
constructs have the potential to target prostate, breast, colon,
ovarian, liver, and head and neck cancers. Depending on the
availability of drug supply, we hope to initiate human clinical
testing for certain of our solid tumor product candidates later this
year.
Efficient Advancement of Potential Future Product Candidates -
Production and Scale Up
We are using our TriKE and
TetraKE platforms with the intent to bring to market multiple
immuno-oncology products that
can treat a range of hematologic malignancies, sarcomas and solid
tumors. The platforms are scalable and we are currently working
with several third parties investigating the optimal expression
system of the TriKEs and
TetraKE constructs which we expect to be part of a process in which
we are able to produce IND-ready moieties in approximately 90-120
days after the construct conceptual design.
After conducting market and competitive research, specific moieties
can then be rapidly advanced into the clinic on our own or through
potential collaborations with larger companies. We are currently evaluating over a
dozen moieties and intend to announce additional clinical
product
candidates.
We believe our TriKEs and
TetraKEs will have the ability, if approved for marketing, to be
used on a stand-alone basis, augment the current monoclonal
antibody therapeutics, or be used in conjunction with more
traditional cancer therapy and potentially overcome certain
limitations of current CAR-T therapy.
Immuno-Oncology Product Candidates
Our TriKE product candidates, GTB-3550 and GTB-C3550, are
single-chain, tri-specific scFv
recombinant fusion proteins composed of the variable regions of the
heavy and light chains (or heavy chain only) of anti-CD16
antibodies, wild-type or a modified form of IL-15 and the variable
regions of the heavy and light chains of an antibody designed to
precisely target a specific tumor antigen. We utilize the NK
stimulating cytokine human IL-15 as a crosslinker between the
two scFvs which is designed to
provide a self-sustaining signal leading to the proliferation and
activation of NK cells thus enhancing their ability to kill cancer
cells mediated by ADCC.
Our TetraKE product candidates are single-chain fusion
proteins composed of human single-domain anti-CD16 antibody,
wild-type IL-15 and the variable regions of the heavy and light
chains of two antibodies that are designed to target two specific
tumor antigens expressed on specific types of cancer cells. An
example of a TetraKE
product candidate is GTB-1615 which is
designed to target EpCAM and
CD133 positive solid tumors. EpCAM is found on many solid tumor cells of
epithelial origin and CD133 is a marker for cancer stem cells.
GTB-1615 is designed to enable a patient’s NK cells to kill
not only the heterogeneous population of cancer cells found in many
solid tumors but also kill the cancer stem cells that can be
responsible for recurrences.
In addition, we have recently terminated further development of
GTB-1550, which targets CD19+ and/or CD22+ hematological
malignancies following completion of the Phase II component of a Phase I/II NHL/ALL trial.
GTB-3550
GTB-3550 is our first TriKE product
candidate. It is a single-chain, tri-specific scFv recombinant fusion protein conjugate composed
of the variable regions of the heavy and light chains of anti-CD16
and anti-CD33 antibodies and a modified form of IL-15. We intend to
study this anti-CD16-IL-15-anti-CD33 TriKE in CD33 positive leukemias, a marker
expressed on tumor cells in AML, MDS, and other hematopoietic
malignancies. CD33 is primarily a myeloid differentiation antigen
with endocytic properties broadly expressed on AML blasts and,
possibly, some leukemic stem cells. CD33 or Siglec-3 (sialic acid binding Ig-like lectin 3, SIGLEC3, SIGLEC3, gp67, p67) is
a transmembrane receptor expressed on cells of myeloid lineage. It
is usually considered myeloid-specific, but it can also be found on
some lymphoid cells. The anti-CD33 antibody fragment that will be
used for these studies was derived from the M195 humanized
anti-CD33 scFV and has been
used in multiple human clinical studies. It has been exploited as
target for therapeutic antibodies for many years. We believe the
recent approval of the antibody-drug conjugate gemtuzumab validates
this targeted approach.
The GTB-3550 IND will focus on AML. These patients typically
receive frontline therapy, usually chemotherapy, including
cytarabine and an anthracycline, a therapy that has not changed in
over 40 years. About half will have relapses and require
alternative therapies. In addition, MDS incidence rates have
dramatically increased in the population of the United States from
3.3 per 100,000 individuals from 2001-2004 to 70 per 100,000
annually, MDS is especially prevalent in elderly patients that have
a median age of 76 years at diagnosis. The survival of patients
with MDS is poor due to decreased eligibility, as a result of
advanced age, for allogeneic hematopoietic cell transplantation
(Allo-HSCT), the only curative
MDS treatment (Cogle CR.
Incidence and Burden of the Myelodysplastic Syndromes.
Curr Hematol Malig
Rep. 2015; 10(3):272-281). We
believe GTB-3550 could serve as a relatively safe, cost-effective
and easy-to-use therapy for resistant/relapsing AML and could also
be combined with chemotherapy as frontline therapy thus targeting
the larger market.
The IND for GTB-3550 was filed in June 2017 by the University of
Minnesota. The FDA requested that additional preclinical toxicology
be conducted prior to initiating clinical trials. The FDA also
requested some additional information and clarifications on the
manufacturing and clinical packages. The requested additional
information and clarifications were completed and incorporated by
us into the IND in eCTD format.
We filed the IND amendment in June 2018 and announced on November
1, 2018 that we had received notification from the FDA that the IND
was open and the Company was authorized to initiate a
first-in-human Phase I study
with GTB-3550 in AML, MDS and severe mastocytosis. We began
the Phase I clinical trial in
January 2020.
GTB-C3550
GTB-C3550 is a next-generation, follow-on, to our lead
TriKE, GTB-3550. GTB-C3550 contains a
modified CD16 moiety which has improved binding characteristics and
enhanced tumor cell killing based on functional assays and animal
models of AML. Using our platform technology, we substituted the
anti-CD16 scFv arm in GTB-3550 with a novel humanized
single-domain anti-CD16 antibody to create this second-generation
molecule which may have improved functionality. Single-domain
antibodies, such as GTB-C3550, typically have several advantages,
including better stability and solubility, more resistance to pH
changes, can better recognize hidden antigenic sites, lack of a VL
portion thus preventing VH/VL mispairing and are suitable for
construction of larger molecules. GTB-C3550 induced a potent
increase in NK cell degranulation, measured by CD107a expression against HL-60 AML tumor targets
when compared to our first-generation TriKE (70.75±3.65% vs. 30.75±5.05%). IFN
production was similarly enhanced (29.2±1.8% vs.
6.55±1.07%). GTB-C3550 also exhibited a robust increase in NK
cell proliferation (57.65±6.05% vs. 20.75±2.55%).
GTB-3550 studies will help inform the development of GTB-C3550
which we expect will de-risk the GTB-C3550 program as data will be
generated to make an informed decision on which, or both, will be
brought into later phase studies.
GTB-1615
GTB-1615 is an example of our first-generation TetraKEs designed for the treatment of solid
tumors. It is a single-chain fusion protein composed of
CD16-IL15-EpCAM-CD133.
EpCAM is found on many solid tumor
cells of epithelial origin and CD133 is a marker for cancer stem
cells. This TetraKE is designed
to target not only the heterogeneous population of cancer cells
found in solid tumors but also the cancer stem cells that are
typically responsible for recurrences. Depending on the
availability of drug supply, we hope to initiate human clinical
testing for certain of our solid tumor product candidates later this
year.
GTB-1550
GTB-1550 is a bispecific scFv
recombinant fusion protein-drug conjugate composed of the variable
regions of the heavy and light chains of anti-CD19 and anti-CD22
antibodies and a modified form of diphtheria toxin (DT390) as its
cytotoxic drug payload. CD19 is a membrane glycoprotein present on
the surface of all stages of
B-lymphocyte development and is also expressed on most B-cell
mature lymphoma cells and leukemia cells. CD22 is a glycoprotein
expressed on B-lineage lymphoid precursors, including precursor
ALL, and often is co-expressed with CD19 on mature B-cell
malignancies such as lymphoma.
GTB-1550 targets cancer cells expressing the CD19 receptor or CD22
receptor or both receptors. When GTB-1550 binds to cancer cells,
the cancer cells internalize GTB-1550 and are killed due to the
action of drug’s cytotoxic diphtheria toxin payload. GTB-1550
has completed a Phase I human
clinical trial in patients with relapsed/refractory B-cell lymphoma
or leukemia.
The initial Phase I study
enrolled 25 patients with mature or precursor B-cell lymphoid
malignancies expressing the CD19 receptor or CD22 receptor or both
receptors. All 25 patients
received at least a single course of therapy. The treatment at the
higher doses produced objective tumor responses with one patient in
continuous partial remission and the second in complete remission.
A Phase I/II trial of GTB-1550
in 18 patients was recently completed in patients with
ALL/NHL.
The FDA-approved clinical trial was conducted at the
University of Minnesota’s
Masonic Cancer Center and concluded in March 2018. Preliminary data
assessment was made in August 2018, and final assessment made June
24, 2020. Based on the lack of efficacy demonstrated by GTB-1550 in
patients evaluated in two Phase
I/II clinical trials (NHL, ALL and CHL), a decision has been made
to terminate further development. We are currently evaluating other
options for GTB-1550.
Our Strategy
Our goal is to be a leader in immuno-oncology therapies targeting a
broad range of indications including hematological malignancies,
sarcoma and solid tumors. Key elements of our strategy are
to:
Rapidly advanced our
Tri-specific Killer Engagers
(TriKEs), GTB-3550 and
GTB-C3550
Our TriKE and TetraKE
product candidates have the potential
to be groundbreaking therapies targeting a broad range of
hematologic malignancies, sarcomas and solid tumors. We are
preparing to study GTB-3550, an
anti-CD16-IL-15-anti-CD33 TriKE
in CD33 positive leukemias, a marker expressed on tumor cells in
AML, MDS and other myeloid malignancies. We began a
Phase I clinical trial in the fourth
quarter of 2019 in patients with relapsed/refractory AML.
The Phase I trial will be a
dose finding study. We expect this will be closely followed
by Phase II trials to determine
the most efficacious dosing and cycles with the aim to maximize
efficacy while minimizing on-target, off-disease adverse
events.
GTB-C3550 contains a humanized single-domain anti-CD16 moiety which
demonstrated improved binding characteristics and enhanced tumor
cell killing based on functional assays and animal models of
AML.
We have designed GTB-3550 and GTB-C3550, if approved for marketing,
to serve as a relatively safe, cost-effective and easy-to-use
therapies for resistant/relapsing AML or MDS which could also be
combined with chemotherapy as frontline therapy thus targeting a
broad AML/MDS market.
GTB-C3550 is a next-generation, follow-on, to our lead
TriKE, GTB-3550. GTB-3550 studies will
help inform the development of GTB-C3550. We believe this will
de-risk the GTB-C3550 program as the data being generated will help
to make informed decisions on which, or both, will be brought into
later phase studies and in which patient
populations.
Utilize our TriKE and TetraKE platform technologies to develop
a robust pipeline of targeted immuno-oncology products targeting a wide range of hematologic
malignancies, sarcomas and solid tumors for development on our own
and through potential collaborations with larger
pharmaceutical companies
We are using our TriKE and
TetraKE platforms with the intent to bring to market multiple,
targeted, off-the-shelf therapies that can treat a range of
hematologic malignancies, sarcomas and solid tumors. The platforms
are scalable and we are currently working with several third
parties investigating the optimal expression system of the
TriKEs and TetraKE constructs which we
expect to be part of a process in which we are able to produce
IND-ready moieties in approximately 90-120 days after the construct
conceptual design. After conducting market and competitive
research, specific moieties can then be rapidly advanced into the
clinic on our own or through potential collaborations with larger
pharmaceutical companies.
We believe our TriKEs and
TetraKEs will have the ability, if approved for marketing, to be
used on a stand-alone basis, augment the current monoclonal
antibody therapeutics, or be used in conjunction with more
traditional cancer therapy and potentially overcome certain
limitations of current CAR-T therapy.
Oncology Markets
B-cell Lymphomas/Leukemias
B-cell lymphoma is a type of cancer that forms in B cells (a type
of immune system cell). B-cell lymphomas may be either indolent
(slow-growing) or aggressive (fast-growing).
Non-Hodgkin lymphoma has an
incidence rate of 19.4 per 100,000 per year and B-cell lymphomas
make up most (about 85%) of NHL in the United States. There are
many different types of B-cell
non-Hodgkin lymphomas. These include Burkitt lymphoma, chronic lymphocytic
leukemia/small lymphocytic lymphoma (CLL/SLL), diffuse large B-cell
lymphoma, follicular lymphoma and mantle cell
lymphoma.
Acute Lymphoblastic Leukemia
ALL is an acute form of leukemia, or cancer of the white blood
cells, characterized by the overproduction and accumulation of
immature white blood cells, known as lymphoblasts. In persons with
ALL, lymphoblasts are overproduced in the bone marrow and
continuously multiply, causing damage and death by inhibiting the
production of normal cells (such as red and white blood cells and
platelets) in the bone marrow and by spreading (infiltrating) to
other organs.
“Acute” is defined by the World Health Organization
standards, in which greater than 20% of the cells in the bone
marrow are blasts. Chronic lymphoblastic leukemia is defined as
having less than 20% blasts in the bone marrow. Acute lymphoblastic
leukemia is seen in both children and adults; the highest incidence
is seen between ages 2 to 3 years (>90 cases per 1 million per
year). ALL is the most common cancer diagnosed in children and
represents approximately 25% of cancer diagnoses among children
younger than 15 years. Among children with ALL, approximately 98%
attain remission, and approximately 85% of patients aged 1 to 18
years with newly diagnosed ALL treated on current regimens are
expected to be long-term event-free survivors, with over 90%
surviving at 5 years.
Multiple Myeloma
Multiple myeloma is a type of cancer that forms in white blood
cells. Multiple myeloma causes cancer cells to accumulate in the
bone marrow, where they crowd out healthy blood cells. Multiple
myeloma is also characterized by destructive lytic bone lesions
(rounded, punched-out areas of bone), diffuse osteoporosis, bone
pain and the production of abnormal proteins which accumulate in
the urine. Anemia is also present in most multiple myeloma patients
at the time of diagnosis and during follow-up. Anemia in multiple
myeloma is multifactorial and is secondary to bone marrow
replacement by malignant plasma cells, chronic inflammation,
relative erythropoietin deficiency and vitamin deficiency. Plasma
cell leukemia, a condition in which plasma cells comprise greater
than 20% of peripheral leukocytes, is typically a terminal stage of
multiple myeloma and is associated with short
survival.
Myeloid Leukemias
Acute Myeloid Leukemia
AML is a heterogeneous hematologic stem cell malignancy in adults
with incidence rate of 4.3% per 100,000 populations. The median age
at the time of diagnosis is 68 years. AML is an aggressive disease
and is fatal without anti-leukemic treatment. AML is the most
common form of adult leukemia in the U.S. These patients will
require frontline therapy, usually chemotherapy including
cytarabine and an anthracycline, a therapy that has not changed in
over 40 years. MDSs are a
heterogeneous group of myeloid neoplasms characterized by
dysplastic features of erythroid/myeloid/megakaryocytic lineages,
progressive bone marrow failure, a varying percentage of blast
cells and enhanced risk to evolve into acute myeloid leukemia. It
is estimated that over 10,000 new cases of MDS are diagnosed each
year and there are minimal treatment options; other estimates have
put this number higher. In addition, the incidence of MDS is rising
for unknown reasons.
Sarcomas
A sarcoma is a type of cancer that develops from certain tissues,
like bone or muscle. Bone and soft tissue sarcomas are the main
types of sarcoma. Soft tissue sarcomas can develop from soft
tissues like fat, muscle, nerves, fibrous tissues, blood vessels or
deep skin tissues. They can be found in any part of the body. Most
of them develop in the arms or legs. They can also be found in the
trunk, head and neck area, internal organs and the area in back of
the abdominal cavity (known as the retroperitoneum). Sarcomas are
not common tumors, and most cancers are the type of tumors called
carcinomas.
The most common types of sarcoma in adults are undifferentiated
pleomorphic sarcoma (previously called malignant fibrous
histiocytoma), liposarcoma and leiomyosarcoma. Certain types occur
more often in certain areas of the body than others. For example,
leiomyosarcomas are the most common abdominal sarcoma, while
liposarcomas and undifferentiated pleomorphic sarcoma are most
common in legs. But pathologists (doctors who specialize in
diagnosing cancers by how they look under the microscope), may not
always agree on the exact type of sarcoma. Sarcomas of uncertain
type are very common (American
Cancer Society, Cancer Facts
& Figures 2019).
Manufacturing
We do not currently own or operate manufacturing facilities for the
production of clinical or commercial quantities of any of
our product candidates. We rely
on a small number of third-party manufacturers to produce our
compounds and expect to continue to do so to meet the preclinical
and clinical requirements of our potential product candidates as well as for
all of our commercial needs. We do not
have long-term agreements with
any of these third parties. We require in our manufacturing and
processing agreements
that all third-party contract
manufacturers and processors produce active pharmaceutical
ingredients and finished products in accordance with the FDA’s cGMPs
and all other applicable laws
and regulations. We maintain confidentiality agreements with potential and existing
manufacturers in order to protect our proprietary rights related to
our drug candidates.
Intellectual Property
We seek to protect our proprietary information by means of United
States and foreign patents, trademarks and copyrights. In addition,
we rely upon trade secret protection and contractual license
agreements to protect certain of our
proprietary information and products. We seek to protect and enhance
proprietary technology, inventions, and improvements that are
commercially important to the development of our business by
seeking, maintaining, and defending patent rights, whether
developed internally or licensed or acquired from third parties. We
also plan to rely on regulatory protection afforded through orphan
drug designations, available regulatory exclusivities and patent
term extensions where available. To achieve this objective, a
strategic focus for us has been to develop our own intellectual
property, while also identifying and licensing patents that provide
protection and serve as an optimal platform to enhance our
intellectual property and technology base.
University of Minnesota Licensed Intellectual Property
We are party to an exclusive worldwide license agreement with the Regents of the University of
Minnesota, to further develop and commercialize cancer therapies
using TriKE technology
developed by researchers at the university to target NK cells to
cancer. Under the terms of the agreement, we receive exclusive rights to conduct
research and to develop, make, use, sell, and import
TriKE technology worldwide for the
treatment of any disease, state or condition in humans. We are
responsible for obtaining all
permits, licenses, authorizations, registrations and regulatory
approvals required or granted by any governmental authority
anywhere in the world that is responsible for the regulation
of products such as the
TriKE technology, including without
limitation the FDA in the United States and the European Agency for the Evaluation of Medicinal Products in the European
Union. We are presently evaluating GTB-3550, our lead
TriKE therapeutic product candidate in a Phase I/II clinical trial. . Under the
agreement, the University of Minnesota
will receive an upfront license fee, royalty fees ranging from 4%
to 6%, minimum annual royalty payments of $0.25 million beginning
in 2022, $2.0 million in 2025, and $5.0 million in 2027 and certain
milestone payments totaling $3.1 million.
The TriKE™ patent estate
licensed from the Regents of the University of Minnesota includes
more than 18 patent applications and the following foundational
patent application:
|
Appl. No.
|
|
Title
|
|
Country
|
|
Status
|
|
PCT Patent Application Number PCT/US2016/055722
|
|
Therapeutic compounds and methods
|
|
Worldwide
|
|
Pending
|
Daniel A. Vallera, Ph.D. Licensed Intellectual
Property
We are party to an exclusive worldwide license agreement with Daniel A. Vallera, Ph.D. and his
co-inventor Jeffrey Lion, or jointly, Dr. Vallera, to further
develop and commercialize DT2219ARL (GTB-1550), a novel therapy for
the treatment of various human cancers. Under the terms of
the agreement, we received
exclusive rights to conduct research and to develop, make, use,
sell, and import DT2219ARL worldwide for the treatment of any
disease, state or condition in humans. We shall be responsible for
obtaining all permits,
licenses, authorizations, registrations and regulatory approvals
required or granted by any governmental authority anywhere in the
world that is responsible for the regulation of products such as DT2219ARL, including without
limitation the FDA in the United States and the European Agency for the Evaluation of Medicinal Products in the European
Union. . Under the agreement,
Dr. Vallera will receive an upfront license fee, royalty fees
ranging from 3% for net sales and 25% of net sublicensing revenues,
and certain milestone payments totaling $1.5
million.
The patent estate licensed from the Dr. Vallera includes more than
16 patent applications and the following issued U.S. patent and
U.S. patent application:
|
Pat./Pub. No.
|
|
Title
|
|
Country
|
|
Status
|
|
U.S. Patent Number 9,371,386
|
|
Methods and compositions for bi-specific targeting of
cd19/cd22
|
|
US
|
|
Issued
|
|
U.S. Patent Application Number 15/187,579
|
|
Methods and compositions for bi-specific targeting of
cd19/cd22
|
|
US
|
|
Pending
|
Employees
As of March 31, 2020, we had two employees. Many of our activities
are outsourced to consultants who provide services to us on a
project basis. As business activities require and capital resources
permit, we will hire additional employees to fulfill our
company’s
needs.
Legal Proceedings
On December 24, 2018, the Empery Funds filed in the N.Y. Supreme
Court, Index No. 656408/2018, alleging causes of action against the
Company for Breach of
Contract, Liquidated
Damages, Damages, and
Indemnification. The claims arose out
of a securities purchase
agreement entered into between the Empery Funds and the Company
pursuant to which the Company issued the Original Securities to the Empery Funds in or
around January 2018. On June 19, 2020, the
Company and the Empery Funds, among others, entered into the
Settlement Agreement resolving all remaining disputes
between the parties pertaining to the Original Securities.
See “Prospectus
Summary—Recent Developments—Settlement with Empery
Funds.”
On August 28, 2019, a complaint was filed in the
Superior Court of California, County
of Los Angeles, West Judicial
District, Santa Monica
Courthouse, Unlimited Civil
Division by Jeffrey Lion and Daniel Vallera. Lion and Vallera are
referred to jointly as the “Plaintiffs”. The complaint was filed against
the Company and its subsidiary Oxis Biotech, Inc. (either of them
or jointly, the “Defendant”). The Plaintiffs allege breach of a license
agreement between the
Plaintiffs and the Defendant entered into on or about September 3,
2015. Lion alleges breach of a consulting agreement between Lion and the
Defendant entered into on or about
September 1, 2015. Vallera alleges breach of a consulting agreement between Vallera and
the Defendant entered into
in or around October, 2018. The
complaint seeks actual damages of $1,670,000, for the fair market
value of the number of shares of the Company’s
common stock that at the time of
judgment represent 15,000,000 shares of such stock as of September
1, 2015, and that the Company issue Lion the number of common
shares the Company’s common stock that at the time of judgment
represent 15,000,000 such shares as of September 1,
2015.
Form and Year of Organization
In 1965, the corporate predecessor of the Company, Diagnostic Data,
Inc., was incorporated in the State of California.
Diagnostic Data, Inc. changed its
incorporation to the State of Delaware in 1972; and changed its
name to DDI Pharmaceuticals, Inc. in 1985. In 1994, DDI
Pharmaceuticals merged with International BioClinical, Inc. and
Bioxytech S.A. and changed its name to OXIS International, Inc. On
July 17, 2017, we amended our certificate of incorporation for the
purpose of changing our name from Oxis International, Inc. to GT
Biopharma, Inc.
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE
GOVERNANCE
The following table sets forth the name, age and position held by
each of our executive officers and directors as of the date of this
prospectus. Directors are elected for a period of one year and
thereafter serve until the next annual meeting at which their
successors are duly elected by the stockholders.
|
Name
|
Age
|
Position
|
|
Anthony J. Cataldo
|
68
|
Chief
Executive Officer and Chairman of the Board
|
|
Steven Weldon
|
44
|
Chief
Financial Officer and Director
|
Anthony J. Cataldo was
appointed Chief Executive Officer and Chairman of the Board on
March 15, 2019. Previously he served as Vice Chairman of the Board
since January 2019. Mr. Cataldo has extensive experience with the
Company, having served on the Board from July 2014 until November
2018, also serving as Chief Executive Officer from November 2014 to
September 2017 and Executive
Chairman of the Board from September 2017 to February 2018 during
that time. Prior to joining the Company, from February 2011 until
June 2013, Mr. Cataldo served as Chairman and CEO/Founder of
Genesis Biopharma, Inc. (now known as Iovance Biotherapeutics,
Inc.). Mr. Cataldo is credited with developing the
Stage Four Cancer treatment for
melanoma known as Lion/Genesis
using assets acquired from the National Cancer Institute (NIH). Mr.
Cataldo also served as non-executive co-chairman of the
board of directors of MultiCell
Technologies, Inc., a supplier of functional, non-tumorigenic
immortalized human hepatocytes from February 2005 until July
2006.
Steven Weldon was appointed
Chief Financial Officer and to the Board on March 20, 2019.
Previously Mr. Weldon was appointed to the Board in September 2014
and as our Chief Financial Officer in November 2014 until October
2018. Mr. Weldon has over 15 years of financial and accounting
experience. Mr. Weldon’s financial background includes
experience in managerial, private accounting and planning. He has
served on the board of several
publicly traded companies as
both chief executive officer and chief financial
officer. Mr. Weldon was appointed as chief financial
officer and as a member of the board of directors of GB Sciences, Inc.
(OTCMKTS:GBLX) in September
2005 and served in both positions until November 2014. Mr. Weldon
also served as chief executive officer of GB Sciences from December
2009, through May 2011, and from April 2012, through March 2014.
For several years, he taught accounting and tax courses to
undergrad students at Florida
Southern College. He received his Bachelor of Science degree and his
Master of Business Administration
from Florida Southern College
and is a licensed Certified
Public Accountant in the State of Florida.
Board Committees, Compensation Committee Interlocks and Insider
Participation
Due to the small number of directors, at the present time the
duties of an Audit
Committee, Nominating and
Governance Committee and Compensation Committee (including with
respect to setting executive officer compensation) are performed by
the Board as a whole. At such time as we have more directors on our
Board, these committees will be reconstituted.
Director Independence
None of our directors qualify as “independent
directors” as defined by Item 407 of Regulation
S-K.
We have elected to use the definition for “director
independence” under the Nasdaq Stock Market’s listing
standards, which defines an “independent director” as
“a person other than an officer or employee of us or its
subsidiaries or any other individual having a relationship, which
in the opinion of our Board, would interfere with the exercise of
independent judgment in carrying out the responsibilities of a
director.” The definition further provides that, among
others, employment of a director by us (or any parent or subsidiary
of ours) at any time during the past three years is considered a
bar to independence regardless of the determination of our
Board.
As a “smaller reporting company” under SEC rules, our named
executive officers for the fiscal year ended December 31, 2019
(collectively, the “Named Executive Officers”) were as
follows:
●
Anthony
J. Cataldo, our current Chief Executive Officer;
●
Steven
Weldon, our current Chief Financial Officer; and
●
Raymond Urbanski, M.D., Ph.D., our former Chief Executive Officer who
resigned from the Company on March 15, 2019.
No
other executive officers received total annual compensation during
the fiscal year ended December 31, 2019 in excess of
$100,000.
Summary Compensation Table
The following table sets forth certain information relating to the
total compensation earned for services rendered to us in
all capacities by our Named Executive
Officers.
|
Name and Principal Position
|
Year
|
|
|
|
All Other Compensation ($)(2)
|
|
|
Anthony J. Cataldo (3)
Chief
Executive Officer
|
2019
|
225,000
|
—
|
1,281,000
|
75,000
|
1,581,000
|
|
Anthony J. Cataldo (3)
Chief
Executive Officer
|
2018
|
190,000
|
—
|
-
|
404,151
|
594,151
|
|
Steven Weldon (4)
|
2019
|
230,000
|
—
|
823,500
|
—
|
1,053,500
|
|
Chief
Financial Officer
|
2018
|
230,000
|
—
|
-
|
—
|
230,000
|
|
Raymond Urbanski, M.D., Ph.D. (5)
|
2019
|
318,000
|
—
|
-
|
—
|
318,000
|
|
Former
Chief Executive Officer
|
2018
|
321,154
|
—
|
7,644,490
|
—
|
7,965,644
|
(1)
The amounts in this
column represent the aggregate grant date fair value of the stock
awards, determined in accordance with Financial Accounting
Standards Board ASC Topic 718. The Company determines the grant
date fair value of the awards by multiplying the number of shares
granted by the closing market price of one share of the
Company’s common stock on the award grant date. These amounts
do not reflect the actual economic value that will be realized by
the Named Executive Officer upon the sale of these
awards.
(2)
The amount in this
column represents compensation earned under a consultant agreement
with the Company described in more detail below under
“—Employment
Arrangements.”
(3)
Mr. Cataldo was
appointed Chief Executive Officer on March 15, 2019. Prior to his
appointment as Chief Executive Officer, Mr. Cataldo provided
services to the Company under a consulting agreement from February
14, 2018. Mr. Cataldo also served as Executive Chairman of the
Board until February 2018 for which service he received salary in
2018.
(4)
Mr. Weldon was
appointed Chief Financial Officer on March 20, 2019.
(5)
Dr. Urbanski
resigned as Chief Executive Officer on March 15, 2019.
Employment Arrangements
On October 18, 2018, the Company entered into a consultant
agreement with Anthony Cataldo (the
“Consulting
Agreement”). The Consulting Agreement terminated in March
2019 in connection with Mr. Cataldo’s appointment as Chief
Executive Officer of the Company. The Consulting Agreement replaced
an earlier consulting
agreement, dated February 14, 2018, and provide for payments of
$25,000 per month to Mr. Cataldo during the term of the
agreement.
On April 1, 2019, the Company entered into a settlement agreement and general release with Mr.
Urbanski (as amended, the “Severance Agreement”) in connection with his
resignation as Chief Executive Officer. Pursuant to the
Severance Agreement, Mr.
Urbanski received a cash severance payment of approximately
$170,000 in two installments. In consideration for the severance
payment, Mr. Urbanski provided a general release of claims in favor
of the Company, including an acknowledgement that Mr. Urbanski was
not entitled to any further compensation, remuneration or benefits
under his executive employment agreement as a result of his resignation from the
Company.
Outstanding Equity Awards at Fiscal Year End
As of December 31, 2019, there were no unexercised options,
unvested stock awards or outstanding equity incentive plan awards
held by our Named Executive Officers.
Director Compensation
The following table summarizes the total compensation we paid to
our non-employee directors for the fiscal year ended December 31,
2019:
|
Name
|
Fees Earned or Paid in Cash ($)
|
|
|
|
|
Dr. John Bonfiglio(1)
|
15,325
|
-
|
-
|
15,325
|
|
Dr. Peter Kiener(1)
|
15,325
|
-
|
-
|
15,325
|
|
Geoffrey Davis(1)
|
15,325
|
-
|
-
|
15,325
|
(1)
Dr. Bonfiglio, Dr.
Kiener and Mr. Davis resigned from the Board on March 20,
2019.
Effective
January 2018, the following annual compensation for non-employee
directors was approved by the Compensation Committee of our
Board:
●
an additional cash
payment of $15,000 for acting as chair of a committee and $5,000
for acting as a member of a committee; and
●
upon approval of a
stock option plan, a grant of options to purchase 150,000 shares of
our common stock, vesting over a three year period (with vesting
accelerating if the Company undergoes a change of control
transaction for cash).
A
director who is one of our employees receives no additional
compensation for his service as a director or as a member of a
committee of the Board.
VOTING SECURITIES AND PRINCIPAL HOLDERS
The following table sets forth certain information regarding
beneficial ownership of our voting securities as of July 28, 2020,
(a) by each person known by us to own beneficially 5% or more of
any class of our voting securities, (b) by each of our Named
Executive Officers, (c) by each of our directors and (d) by
all our current executive officers and
directors as a group. As of July 28, 2020, there were 76,560,862
shares of our common stock
issued and outstanding. Shares
of common stock subject to
stock options, preferred stock and convertible notes and debentures
that are currently exercisable or convertible within 60 days of
July 28, 2020 are deemed to be outstanding for purposes of
computing the percentage ownership of that person but are not
treated as outstanding for computing the percentage ownership of
any other person. Unless indicated below, the persons and entities
named in the table have sole voting and sole investment power with
respect to all shares
beneficially owned, subject to community property laws where
applicable. Except as otherwise indicated, the address of each
stockholder is c/o GT Biopharma, Inc. at 9350 Wilshire Blvd., Suite
203, Beverly Hills, CA 90212.
|
Name
and Address of Beneficial Owner
|
Shares
of Common Stock Beneficially Owned
|
Percentage
of Class Outstanding
|
Shares of Series J-1 Preferred Stock Beneficially
Owned(1)
|
Percentage
of Class Outstanding
|
|
|
|
|
|
|
|
Security Ownership of Certain Beneficial
Owners:
|
|
|
|
|
|
Alpha
Capital Anstalt(2)
|
7,648,430(3)
|
9.99%(4)
|
—
|
|
|
Bristol Capital, LLC(5)
|
—(6)
|
—
|
1,575,324
|
66.9%
|
|
Bristol Investment Fund, Ltd.(5)
|
6,619,779(7)
|
9.99%(8)
|
778,224
|
33.1%
|
|
James
Heavener(9)
|
7,648,430(10)(15)
|
9.99%(16)
|
—
|
—
|
|
Adam
Kasower
|
7,625,485(10)
|
9.96%
|
|
|
|
Bigger
Capital Fund, LP(11)
|
4,500,000(10)
|
5.88%
|
—
|
—
|
|
District 2 Capital Fund LP(12)
|
4,289,077(10)
|
5.60%
|
—
|
—
|
|
GT Bio
Partners LLC(13)
|
7,500,000(10)
|
9.80%
|
—
|
—
|
|
Kevin
Young
|
5,000,000(10)
|
6.53%
|
—
|
—
|
|
Red
Mango Enterprises Limited(14)
|
7,648,430(10)(15)
|
9.99%(16)
|
—
|
—
|
|
The Rosalinde
and Arthur Gilbert
Foundation(17)
|
7,648,430(10)(15)
|
9.99%(16)
|
—
|
—
|
|
The
RSZ Trust(18)
|
5,938,566(10)
|
7.76%
|
—
|
—
|
|
|
|
|
|
|
|
Security Ownership of Management and Directors:
|
|
|
|
|
|
Anthony J.
Cataldo
|
7,013,345
|
9.16%
|
—
|
—
|
|
Steven
Weldon
|
4,500,000
|
5.89%
|
—
|
—
|
|
Executive
officers and directors as a group — 2
people
|
11,513,345
|
15.05%
|
—
|
—
|
(1)
Each share of our
Series J-1 Preferred Stock is convertible into five shares of our
common stock at an effective conversion price of $0.20 per share.
Shares of the Series J-1 Preferred Stock have the same voting
rights as shares of our common stock with the holders of the Series
J-1 Preferred Stock entitled to vote on an as-converted-to-common
stock basis, subject to the certain beneficial ownership
limitations, together with the holders of our common stock on all
matters presented to our stockholders. See “Description of Capital Stock—Preferred
Stock—Series J-1 Preferred Stock” for more
information about the terms of our Series J-1 Preferred
Stock.
(2)
The address of
Alpha Capital Anstalt (“Alpha Capital”) is Lettstrasse
32, FL-9490 Vaduz, Furstentums, Liechtenstein. We have been advised
Konrad Ackermann exercises voting and investment power over
securities held by Alpha Capital.
(3)
As reported on
Schedule 13G/A filed with the SEC on January 16, 2020, Alpha
Capital holds shares of our common stock plus other securities
(including certain of the Applicable Notes) that are convertible or
exercisable for shares of our common stock only if such conversion
or exercise does not result in Alpha Capital (together with its
affiliates) holding more than 9.99% of our outstanding shares of
common stock. The full conversion or exercise of such securities of
the Company held by Alpha Capital would exceed such beneficial
ownership limitation. This represents the maximum number of shares
of common stock that Alpha Capital could beneficially own as of
July 28, 2020.
(4)
Calculated based on
the maximum number of shares of common stock that Alpha Capital
could have beneficially owned on July 28, 2020 following conversion
or exercise of securities held by Alpha Capital, subject to the
beneficial ownership limitation described in note (3)
above.
(5)
Paul Kessler, as
manager of Bristol Capital Advisors, LLC, the investment advisor to
Bristol Investment Fund, Ltd. (“BIF”), has voting and
investment control over the securities held by BIF. Mr. Kessler, as
manager of Bristol Capital, LLC, also has voting and investment
control over the securities held by Bristol Capital. Mr. Kessler
disclaims beneficial ownership of these securities except to the
extent of his pecuniary interest therein. The address of Bristol
Capital Advisors, LLC is 662 N. Sepulveda Blvd., Suite 300, Los
Angeles, California 90049.
(6)
As reported on
Schedule 13G filed with the SEC on June 10, 2020. Excludes shares
of our common stock that may be issued upon conversion of the
Series J-1 Preferred Stock held by Bristol Capital. Such Series J-1
Preferred Stock may be converted into shares of our common stock
only if such conversion does not result in Bristol Capital
(together with its affiliates, including BIF) holding more than
9.99% of our outstanding shares of common stock.
(7)
As reported on
Schedule 13G filed with the SEC on June 10, 2020. As disclosed in
the Schedule 13G, BIF also holds Series J-1 Preferred Stock and
convertible notes which may be converted into shares of our common
stock only if such conversion does not result in BIF (together with
its affiliates, including Bristol Capital) holding more than 9.99%
of our outstanding shares of common stock. The full conversion of
such securities would exceed such beneficial ownership limitation.
As of July 28, 2020, the maximum number of shares of common stock
that BIF could beneficially own was 7,648,430 shares.
(8)
Calculated based on
the maximum number of shares of common stock that BIF could have
beneficially owned on July 28, 2020 following conversion of the
Series J-1 Preferred Stock or convertible notes, subject to the
beneficial ownership limitation described in note (7)
above.
(9)
The address of Mr.
Heavener is 3300 University Blvd, Suite 218 Winter Park, FL
32792.
(10)
Represents or
includes shares of common stock that may be issuable to the
stockholder upon conversion of certain convertible notes, including
certain of the Applicable Notes, and excludes additional shares of
common stock that may be issuable to the stockholder (i) in lieu of
cash payments of interest or (ii) in connection with any default
amounts. Such convertible notes are only convertible if such
conversion does not result in the stockholder (together with its
affiliates) holding more than 9.99% of our outstanding shares of
common stock.
(11)
We have been
advised that Michael Bigger exercises voting and investment power
over the securities held by Bigger Capital Fund, LP.
(12)
We have been
advised that Eric H Schlanger exercises voting and investment power
over the securities held by District 2 Capital Fund
LP.
(13)
We have been
advised that Philip G. Werthman exercises voting and investment
power over the securities held by of GT Bio Partners
LLC.
(14)
We have been
advised that Chi Kan Tang exercises voting and investment power
over the securities held by Red Mango Enterprises
Limited.
(15)
The full conversion
or exercise of convertible notes or other securities convertible
into, or exercisable for, our common stock held by the stockholder
would exceed the beneficial ownership limitation described in note
(10) above. This represents the maximum number of shares of common
stock that the stockholder could beneficially own as of July 28,
2020.
(16)
Calculated based on
the maximum number of shares of common stock that the stockholder
could have beneficially owned on July 28, 2020 following conversion
or exercise of convertible notes or other securities convertible
into, or exercisable for, our common held by the stockholder,
subject to the beneficial ownership limitation described in note
(10) above.
(17)
We have been
advised that Martin H. Blank exercises voting and investment power
over the securities held by The Rosalinde and Arthur Gilbert
Foundation.
(18)
We have been
advised that Richard exercises voting and investment power over the
securities held by RSZ Trust.
SELLING STOCKHOLDERS
The Registered Shares being offered by the Selling Stockholders are
those that (a) were issued to a Selling Stockholder pursuant to the
terms of a consulting agreement or (b) that may be issued to
certain of the Selling Stockholders either (i) upon conversion of
the Applicable Notes, or (ii) at the option of the Selling
Stockholders as holders of the Applicable Notes, in lieu of cash
payments of interest on the Applicable Notes based upon the then
current conversion price for the Applicable Notes. We are
registering the Registered Shares in order to permit the Selling
Stockholders to offer the shares for resale from time to time.
Except for ownership of the Applicable Notes or other securities of
the Company issued in connection with prior financing transactions
and certain consulting arrangements, the Selling Stockholders have
not had any material relationship with us within the past three
years.
The table below lists the Selling Stockholders and other
information regarding the beneficial ownership of our
common stock by each of the Selling
Stockholders. The second column lists the number of shares
of common stock beneficially
owned by each Selling Stockholder, based on its ownership as of
July 28, 2020. The third column lists the shares of
common stock being offered by this
prospectus by the Selling Stockholders and does not take in account
any limitations on conversion of the Applicable Notes or issuance
of common
stock.
In accordance with the terms of the Registration Rights
Agreements related to the Applicable Notes, this prospectus
generally covers the resale of at least the sum of the number of
shares of common stock issued
upon conversion of the Applicable Notes issued pursuant to the
applicable securities purchase
agreement as of the trading day immediately preceding the date the
registration statement is initially filed with the SEC plus an
additional approximately 2.8 million shares of common stock that may be issued in connection with
interest payments under the Applicable Notes. The fourth column assumes the sale of
all of the shares offered by the
Selling Stockholders pursuant to this
prospectus.
Under the terms of the Applicable Notes, a Selling Stockholder may
not convert the Applicable Notes to the extent such exercise would
cause such Selling Stockholder, together with its affiliates, to
beneficially own a number of shares of our common stock which would exceed 9.99% of our then
outstanding shares of common
stock following such exercise. The number of shares in the second
column does not reflect these limitations. The Selling Stockholders
may sell all, some or none of
their shares in this offering. See “Plan of
Distribution.”
|
|
Shares of Common Stock Beneficially Owned after the
Offering
|
|
Name of Selling Shareholder
|
Number of Shares of Common Stock Owned Prior to
Offering(1)
|
Maximum Number of Shares of Common Stock to be Sold Pursuant to
this Prospectus
|
|
|
|
Adam
Kasower
|
7,625,485(2)
|
1,375,000
|
6,375,485(2)
|
8.33%
|
|
Alpha Capital Anstalt(3)
|
7,648,430(4)
|
1,100,000
|
7,648,430(4)
|
9.99%(5)
|
|
Bigger Capital Fund, LP(6)
|
4,500,000(2)
|
3,575,000
|
3,250,000(2)
|
4.24%
|
|
Brannon Family Office LLLP(7)
|
585,000(2)*
|
643,500
|
*
|
*
|
|
Christopher
and Lorraine Basta JTWROS
|
375,000(2)*
|
412,500
|
*
|
*
|
|
District 2 Capital Fund LP(8)
|
4,289,077(2)
|
1,375,000
|
3,039,077(2)
|
3.97%
|
|
Edward
Flanagan
|
250,000(2)*
|
275,000
|
*
|
*
|
|
Edwin
Ting
|
1,000,000(2)*
|
1,100,000
|
*
|
*
|
|
EMLL Group LLC(7)
|
1,086,429*
|
1,086,429
|
*
|
*
|
|
GT Bio Partners LLC(9)
|
7,500,000(2)*
|
7,975,000
|
*
|
*
|
|
Houngly
Nguyen
|
250,000(2)*
|
275,000
|
*
|
*
|
|
Kevin
Young
|
5,000,000(2)*
|
5,500,000
|
*
|
*
|
|
Mark
Flanagan
|
200,000(2)*
|
220,000
|
*
|
*
|
|
Matthew
J. Gantz Irrevocable Trust DTD 4/20/06
|
375,000(2)*
|
412,500
|
*
|
*
|
|
Philip G. Werthman Trust(10)
|
250,000(2)*
|
275,000
|
*
|
*
|
|
Red Mango Enterprises Limited(11)
|
7,648,430(2)(12)
|
4,125,000
|
5,987,663(2)
|
7.82%
|
|
Riley
Flanagan
|
500,000*
|
550,000
|
*
|
*
|
|
The Rosalinde and Arthur Gilbert
Foundation(13)
|
7,648,430(2)(12)
|
1,100,000
|
7,648,430(2)(12)
|
9.99%(14)
|
|
The RSZ Trust(15)
|
5,938,566(2)
|
550,000
|
5,438,566(2)
|
7.10%
|
_______________
*It is unknown to the Company
whether or not the Selling Stockholder holds shares of
common stock other than those being
registered.
(1)
Excludes additional shares of common stock that may be issuable to
the Selling Stockholders (i) in lieu of cash payments of interest
or (ii) in connection with any default amounts, including
approximately 2.8 million shares of common stock that are
registered pursuant to this registration statement and that may be
issued in connection with interest payments under the Applicable
Notes.
(2)
Represents or includes shares of common stock that may be issuable
to the Selling Stockholder upon conversion of certain convertible
notes, including certain of the Applicable Notes. Such convertible
notes are only convertible if such conversion does not result in
the Selling Stockholder (together with its affiliates) holding more
than 9.99% of our outstanding shares of common stock.
(3) We have been advised that Konrad Ackermann exercises voting and investment
power over the shares of common
stock registered on behalf of the Alpha Capital pursuant to this registration
statement.
(4) See note (3) to the beneficial ownership table
included under “Voting Securities and
Principal Holders” for
additional information.
(5) See note (4) to the beneficial ownership table
included under “Voting Securities and
Principal Holders” for
additional information.
(6) We have been advised that Michael Bigger
exercises voting and investment power over the shares of
common stock registered on behalf of
Bigger Capital Fund, LP pursuant to this registration
statement.
(7) We have been advised that Dwain Brannon
exercises voting and investment power over the shares of
common stock registered on behalf of
Brannon Family Office LLLP and EMLL Group, LLC pursuant to this
registration statement.
(8) We have been advised that Eric H Schlanger
exercises voting and investment power over the shares of
common stock registered on behalf
of District 2 Capital Fund LP
pursuant to this registration statement.
(9) We have been advised that Philip G. Werthman
exercises voting and investment power over the shares of
common stock registered on behalf of
GT Bio Partners LLC pursuant to this registration
statement.
(10) We have been advised that Philip G. Werthman
exercises voting and investment power over the shares of
common stock registered on behalf of
the Phillip G. Werthman Trust pursuant to this registration
statement.
(11) We have been advised that Chi Kan Tang
exercises voting and investment power over the shares of
common stock registered on behalf of
Red Mango Enterprises Limited pursuant to this registration
statement.
(12)
The full conversion or exercise of securities held by the Selling
Stockholder would exceed the beneficial ownership limitation
described in note (2) above. This represents the maximum number of
shares of common stock that Selling Stockholder could beneficially
own as of July 28, 2020.
(13) We have been advised that Martin H. Blank
exercises voting and investment power over the shares of
common stock registered on behalf
of The Rosalinde and Arthur
Gilbert Foundation pursuant to this registration
statement.
(14)
Calculated based on the maximum number of shares of common stock
that the Selling Stockholder could have beneficially owned on July
28, 2020 following conversion or exercise of securities held by the
Selling Stockholder, subject to the beneficial ownership limitation
described in note (2) above.
(15) We have been advised that Richard Ziman
exercises voting and investment power over the shares of
common stock registered on behalf of
the RSZ Trust pursuant to this
registration statement.
We are registering 31,924,929 shares of our common stock for possible sale by the Selling
Stockholders. We will not receive any of the proceeds from the sale
by the Selling Stockholders of the shares of common stock. We will bear all fees and expenses incident to our obligation
to register the shares of common stock.
The Selling Stockholders may sell all or a portion of the shares of
common stock beneficially owned by
them and offered hereby from time to time directly or through one
or more underwriters, broker-dealers or agents. If the shares
of common stock are sold
through underwriters or broker-dealers, the Selling Stockholders
will be responsible for underwriting discounts or commissions or
agent’s commissions. The shares of common stock may be sold in one or more
transactions at fixed prices, at prevailing market prices at the
time of the sale, at varying prices determined at the time of sale,
or at negotiated prices. These sales may be effected in
transactions, which may involve crosses or block transactions,
pursuant to one or more of the following
methods:
●
on
any national securities exchange or quotation service on which the
securities may be listed or quoted at the time of
sale;
●
in
the over-the-counter market;
●
in
transactions otherwise than on these exchanges or systems or in the
over-the-counter market;
●
through
the writing of options, whether such options are listed on an
options exchange or otherwise;
●
ordinary
brokerage transactions and transactions in which the broker-dealer
solicits purchasers;
●
block
trades in which the broker-dealer will attempt to sell the shares
as agent but may position and resell a portion of the block as
principal to facilitate the transaction;
●
purchases
by a broker-dealer as principal and resale by the broker-dealer for
its account;
●
an
exchange distribution in accordance with the rules of the
applicable exchange;
●
privately
negotiated transactions;
●
short sales effected after the effective date of
this registration
statement;
●
sales
pursuant to Rule 144;
●
broker-dealers
may agree with the Selling Stockholders to sell a specified number
of such shares at a stipulated price per share;
●
a
combination of any such methods of sale; and
●
any
other method permitted pursuant to applicable law.
If the Selling Stockholders effect such transactions by selling
shares of common stock to or
through underwriters, broker-dealers or agents, such underwriters,
broker-dealers or agents may receive commissions in the form of
discounts, concessions or commissions from the Selling Stockholders
or commissions from purchasers of the shares of common stock for whom they may act as agent or to
whom they may sell as principal (which discounts, concessions or
commissions as to particular underwriters, broker-dealers or agents
may be in excess of those customary in the types of transactions
involved). In connection with sales of the shares of
common stock or otherwise, the Selling
Stockholders may enter into hedging transactions with
broker-dealers, which may in turn engage in short sales of the
shares of common stock in the
course of hedging in positions they assume. The Selling
Stockholders may also sell shares of common stock short and deliver shares of
common stock covered by this
prospectus to close out short positions and to return borrowed
shares in connection with such short sales. The Selling
Stockholders may also loan or pledge shares of common stock to broker-dealers that in turn may
sell such shares.
The Selling Stockholders may pledge or grant a security interest in
some or all of the shares
of common stock or
Notes owned by them and, if they
default in the performance of their secured obligations, the
pledgees or secured parties may offer and sell the shares of
common stock from time to time
pursuant to this prospectus or any amendment to this prospectus
under Rule 424(b)(3) or other applicable provision of the
Securities Act, amending, if necessary, the list of Selling
Stockholders to include the pledgee, transferee or other successors
in interest as Selling Stockholders under this prospectus. The
Selling Stockholders also may transfer and donate the shares
of common stock in other
circumstances in which case the transferees, donees, pledgees or
other successors in interest will be the selling beneficial owners
for purposes of this prospectus.
The Selling Stockholders and any broker-dealer participating in the
distribution of the shares of common stock may be deemed to be
“underwriters” within the meaning of the Securities
Act, and any commission paid, or any discounts or concessions
allowed to, any such broker-dealer may be deemed to be underwriting
commissions or discounts under the Securities Act. At the time a
particular offering of the shares of common stock is made, a prospectus supplement, if
required, will be distributed which will set forth the aggregate
amount of shares of common
stock being offered and the terms of the offering, including the
name or names of any broker-dealers or agents, any discounts,
commissions and other terms constituting compensation from the
Selling Stockholders and any discounts, commissions or concessions
allowed or re-allowed or paid to
broker-dealers.
Under the securities laws of some states, the shares of
common stock may be sold in such
states only through registered or licensed brokers or dealers. In
addition, in some states the shares of common stock may not be sold unless such shares
have been registered or qualified for sale in such state or an
exemption from registration or qualification is available and is
complied with.
There can be no assurance that any Selling Stockholder will sell
any or all of the shares
of common stock registered
pursuant to the registration statement, of which this prospectus
forms a part.
The Selling Stockholders and any other person participating in such
distribution will be subject to applicable provisions of the
Exchange Act and the rules and regulations thereunder, including,
without limitation, Regulation M of the Exchange Act, which may
limit the timing of purchases and sales of any of the shares
of common stock by the Selling
Stockholders and any other participating person. Regulation M may
also restrict the ability of any person engaged in the distribution
of the shares of common stock
to engage in market-making activities with respect to the shares
of common stock.
All of the foregoing may affect the
marketability of the shares of common stock and the ability of any person or
entity to engage in market-making activities with respect to the
shares of common
stock.
We will pay all expenses of the
registration of the shares of common stock pursuant to the Registration Rights
Agreement, estimated to be $10,000 in total, including, without
limitation, SEC filing fees and expenses of compliance with state
securities or “blue sky” laws; provided, however, that
a Selling Stockholder will pay all underwriting discounts and selling
commissions, if any. We will indemnify the Selling Stockholders
against liabilities, including some liabilities under the
Securities Act, in accordance with the Registration Rights
Agreement, or the Selling Stockholders will be entitled to
contribution. We may be indemnified by the Selling Stockholders
against civil liabilities, including liabilities under the
Securities Act, that may arise from any written information
furnished to us by the Selling Stockholder specifically for use in
this prospectus, in accordance with the Registration Rights
Agreement, or we may be entitled to
contribution.
Once sold under the registration statement, of which this
prospectus forms a part, the shares of common stock will be freely tradable in the hands
of persons other than our affiliates.
DESCRIPTION OF CAPITAL STOCK
The following description of our capital stock, together with any
additional information we include in any applicable prospectus
supplement or any related free writing prospectus, summarizes the
material terms and provisions of our capital stock. For the
complete terms of our capital stock, please refer to our
certificate of incorporation bylaws that are incorporated by
reference into the registration statement of which this prospectus
is a part or may be incorporated by reference in this prospectus or
any applicable prospectus supplement. The terms of these securities
may also be affected by the DGCL. The summary below and that
contained in any applicable prospectus supplement or any related
free writing prospectus are qualified in their entirety by
reference to our certificate of incorporation and
bylaws.
General
As of the date of this prospectus, our authorized capital stock
consists of 750.0 million shares of common stock, par value $0.001 per share, and 15.0
million shares of preferred stock, par value $0.001 per share. As
of July 28, 2020, there were 76,560,862 shares of our
common stock, 96,230 shares of Series
C Preferred Stock and 2,353,548 shares of Series J-1 Preferred
Stock issued and outstanding.
Common Stock
Holders of our common stock are
entitled to one vote for each share of common stock held of record for the election of
directors and on all matters
submitted to a vote of stockholders. Holders of our
common stock are entitled to receive
dividends ratably, if any, as may be declared by the Board out of
legally available funds, subject to any preferential dividend
rights of any preferred stock then outstanding. In the event of our
dissolution, liquidation or winding up, holders of our
common stock are entitled to share
ratably in our net assets legally available after the payment
of all of our debts and other
liabilities, subject to the liquidation preferences of any
preferred stock then outstanding. Holders of our
common stock have no preemptive,
subscription, redemption or conversion rights. The rights,
preferences and privileges of holders of common stock are subject to, and may be adversely
affected by, the rights of the holders of shares of any series of
preferred stock currently outstanding or that we may designate and
issue in the future. All
outstanding shares of our common stock are fully paid and non-assessable.
Except as described below in “Anti-Takeover Provisions Under Our Charter and
Bylaws and Delaware Law,” holders of a majority of the
outstanding shares of stock entitled to vote shall constitute a
quorum for the transaction of business, and a vote of the majority
of the voting power represented at such meeting at which a quorum
is generally required to take action under our certificate of
incorporation and bylaws.
Preferred Stock
Our Board is authorized, without action by the stockholders, to
designate and issue up to 15.0 million shares of preferred stock in
one or more series. In the past the Board has designated series
lettered A through J-1 and issued shares in those series. As of the
date of this prospectus, only preferred shares in the series
designated C and J-1 have shares issued and outstanding. Our Board
can fix or alter the rights, preferences and privileges of the
shares of each series and any of its qualifications, limitations or
restrictions, including dividend rights, conversion rights, voting
rights, terms of redemption, liquidation preferences and the number
of shares constituting a class or series. The issuance of preferred
stock could, under certain circumstances, result in one or more of
the following adverse effects:
●
decreasing the
market price of our common stock;
●
restricting
dividends on our common stock;
●
diluting the voting
power of our common stock;
●
impairing the
liquidation rights of our common stock; or
●
delaying or
preventing a change in control of us without further action by our
stockholders.
Our Board will make any determination to issue such shares based on
its judgment as to our best interests and the best interests of our
stockholders.
Series C Preferred Stock
For a discussion of the terms of our Series C Preferred Stock, see
Note 7 to our audited financial statements, Stockholders’
Equity.
Series J-1 Preferred Stock
Shares of our Series J-1 Preferred Stock are convertible at any
time, at the option of the holders, into shares of our common stock
at an effective conversion price of $0.20 per share, subject to
adjustment for, among other things, stock dividends, stock splits,
combinations, reclassifications of our capital stock and mergers or
consolidations, and subject to a “blocker provision”
which prohibits conversion if such conversion would result in the
holder being the beneficial owner of in excess of 9.99% of our
common stock. Shares of our Series J-1 Preferred Stock have the
same voting rights a shares of our common stock, with the holders
of the Series J-1 Preferred Stock entitled to vote on an
as-converted-to-common stock basis, subject to the “blocker
provision” described above, together with the holders of our
common stock on all matters presented to our stockholders. The
Series J-1 Preferred Stock are not entitled to any dividends
(unless specifically declared by our Board), but will participate
on an as-converted-to-common-stock basis in any dividends to the
holders of our common stock. In the event of our dissolution,
liquidation or winding up, the holders of our Series J-1 Preferred
Stock will be on parity with the holders of our common stock and
will participate, on a on an as-converted-to-common stock basis, in
any distribution to holders of our common stock.
Anti-Takeover Provisions Under Our Charter and Bylaws and Delaware
Law
Certain provisions of Delaware law, our certificate of
incorporation and our bylaws contain provisions that could have the
effect of delaying, deferring or discouraging another party from
acquiring control of us. These provisions, which are summarized
below, may have the effect of discouraging coercive takeover
practices and inadequate takeover bids. These provisions are also
designed, in part, to encourage persons seeking to acquire control
of us to first negotiate with our Board. We believe that the
benefits of increased protection of our potential ability to
negotiate with an unfriendly or unsolicited acquirer outweigh the
disadvantages of discouraging a proposal to acquire us because
negotiation of these proposals could result in an improvement of
their terms.
Amended and Restated Certificate of Incorporation
Undesignated Preferred Stock.
Our Board has the ability to issue preferred stock with voting or
other rights or preferences that could impede the success of any
attempt to change control of us. These and other provisions may
have the effect of deferring hostile takeovers or delaying changes
in control or management of our company.
Special Meetings of Stockholders. Our bylaws provide that special meetings of our
stockholders may be called only by our Chairman of the Board, our
president or our Board, thus prohibiting a stockholder from calling
a special meeting. This provision might delay the ability of our
stockholders to force consideration of a proposal or for
stockholders controlling a majority of our capital stock to take
any action, including the removal of directors.
Board Vacancies Filled Only by Majority of
Directors. Vacancies and newly
created seats on our Board may be filled only by a majority of the
directors then in office. Only our Board may determine the number
of directors on our board. The
inability of stockholders to determine the number of directors or
to fill vacancies or newly created seats on our Board makes it more
difficult to change the composition of our Board, but these
provisions promote a continuity of existing
management.
No Cumulative Voting. The DGCL
provides that stockholders are not entitled to the right to
cumulate votes in the election of directors unless our certificate
of incorporation provides otherwise. Our certificate of
incorporation and bylaws do not expressly provide for cumulative
voting.
Directors Removed Only by Special Meeting of
Stockholders. A director can be
removed only by the affirmative vote of a majority of the votes of
the issued and outstanding stock entitled to vote for the election
of directors of the corporation given at a special meeting of the
stockholders called and held for this purpose.
Amendment of Charter Provisions. In order to amend certain of the above
provisions in our certificate of incorporation and our bylaws, the
Board is expressly authorized to adopt, alter or repeal the bylaws,
subject to the rights of the stockholders entitled to vote.
Stockholders can vote at any stockholder meeting and repeal, alter,
or amend the bylaws by the affirmative vote of a majority of the
stockholders entitled to vote in such meeting.
Delaware Anti-takeover
Statute
We are subject to Section 203 of the DGCL. In general,
Section 203 prohibits a publicly held Delaware corporation
from engaging in a “business combination” with an
“interested stockholder” for a period of three years
after the date of the transaction in which the person became an
interest stockholder, unless the business combination is approved
in a prescribed manner. A “business combination”
includes mergers, asset sales and other transactions in which the
interested stockholder receives or could receive a financial
benefit on other than a pro rata basis with other stockholders. An
“interested stockholder” is a person who, together with
affiliates and associates, owns, or within three years did own, 15%
or more of the corporation’s outstanding voting stock. This
provision has an anti-takeover effect with respect to transactions
not approved in advance by our Board, including discouraging
takeover attempts that might result in a premium over the market
price for the shares of our market price. With approval of our
stockholders, we could amend our amended and restated certificate
of incorporation in the future to avoid the restrictions imposed by
this anti-takeover law.
The provisions of Delaware law and our amended and restated
certificate of incorporation could have the effect of discouraging
others from attempting hostile takeovers and, as a consequence,
they may also inhibit temporary fluctuations in the market price of
our common stock that often
result from actual or rumored hostile takeover attempts. These
provisions may also have the effect of preventing changes in our
management. It is possible that these provisions could make it more
difficult to accomplish transactions that stockholders may
otherwise deem to be in their best interests.
Transfer Agent and Registrar
Our transfer agent and registrar for our capital stock is
Computershare. The transfer
agent’s address is 8742 Lucent Blvd., Suite 225, Highland Ranch, CO 80129, and its
telephone number is (303) 262-0600.
Existing Trading Markets
Our common stock is quoted on
the OTCQB, one of the OTC
Markets Group over-the-counter markets, under the trading symbol
“GTBP.” The closing sale price of our
common stock on the OTCQB on July 13,
2020, was $0.16per share. Our common stock is also quoted on several
European-based exchanges including
Berlin (GTBP.BE), Frankfurt
(GTBP.DE), the Euronext (GTBP.NX) and Paris (GTBP.PA).
The validity of the shares of common stock offered by this prospectus will be
passed upon for us by Baker & McKenzie LLP, Houston,
Texas.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES
ACT LIABILITIES
Insofar as indemnification for liabilities arising under the
Securities Act may be permitted to directors, officers or persons
controlling the registrant pursuant to the foregoing provisions,
the registrant has been informed that in the opinion of the SEC
such indemnification is against public policy as expressed in the
Securities Act and is therefore unenforceable.
The financial statements of GT Biopharma, Inc. at December 31, 2019
and 2018, and for each of the two years in the period ending
December 31, 2019, appearing in this prospectus have been audited
by Seligson & Giannattasio, LLP, an independent registered
public accounting firm, as set forth in their report thereon
appearing elsewhere herein, and are included in reliance upon such
report given on the authority of such firm as experts in accounting
and auditing.
WHERE YOU
CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-1 under the
Securities Act with the SEC with respect to the resale of the
Registered Shares. This prospectus was filed as a part of that
registration statement but does not contain all of the information contained in the
registration statement and exhibits. Reference is thus made to the
omitted information. Statements made in this prospectus are
summaries of the material terms of contracts, agreements and documents and are not necessarily
complete; however, all
information we considered material has been disclosed. Reference is
made to each exhibit for a more complete description of the matters
involved and these statements are qualified in their entirety by
the reference. The SEC also maintains a web site
(http://www.sec.gov) that
contains this filed registration statement, reports and other
information regarding us that we have filed electronically with the
SEC. For more information pertaining to our company and the sale or resale of an aggregate of
31,924,929 shares of common
stock, reference is made to the registration
statement.
INDEX TO FINANCIAL
STATEMENTS
Audited Consolidated Financial Statements of GT Biopharma,
Inc.
|
Report of Independent Registered
Public Accounting
Firm
|
F-2
|
|
Consolidated Balance Sheets as of
December 31, 2019 and
2018
|
F-3
|
|
Consolidated Statement of Operations For Years Ended December 31,
2019 and 2018
|
F-4
|
|
Consolidated Statement of Stockholders’ Equity For Years
Ended December 31, 2019 and 2018
|
F-5
|
|
Consolidated Statement of Cash Flows For Years Ended December 31,
2019 and 2018
|
F-6
|
|
Notes to Consolidated Financial
Statements
|
F-7
|
Unaudited Consolidated Financial Statements of GT Biopharma,
Inc.
|
Consolidated Balance Sheets as of March 31, 2020 and December 31,
2019
|
F-20
|
|
Consolidated Statements of
Operations for the three months ended March 31, 2020 and
2019
|
F-21
|
|
Consolidated Statements of Cash
Flows for the three months ended March 31, 2020 and
2019
|
F-22
|
|
Condensed Notes to Consolidated
Financial
Statements
|
F-23
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING
FIRM
To the Board of Directors and Stockholders of GT Biopharma,
Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of GT
Biopharma, Inc. and subsidiaries (the “Company”) as of December 31, 2019 and 2018,
and the related consolidated statements of operations,
stockholders’ equity and cash flows for each of the years in
the two-year period ended December 31, 2019, and the related
notes (collectively referred to as the
financial statements). In our opinion, the consolidated financial
statements present fairly, in all material respects, the consolidated financial
position of the Company as of December 31, 2019 and 2018 and the
consolidated results of its operations and its consolidated cash
flows for each of the years in the two-year period ended December
31, 2019, in conformity with accounting principles generally
accepted in the United States of America.
Basis of Opinion
These financial statements are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on the Company’s consolidated financial statements
based on our audits. We are a public accounting firm registered
with the Public Company Accounting Oversight Board (United States)
(“PCAOB”) and are required to be independent with
respect to the Company in accordance with the U.S. federal
securities laws and the applicable rules and regulations of the
Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the
PCAOB. Those standards require that we plan and perform the audit
to obtain reasonable assurance about whether the consolidated
financial statements are free of material misstatement, whether due
to error or fraud. The Company is not required to have, nor were we
engaged to perform, an audit of its internal control over financial
reporting. As part of our audits we are required to obtain an
understanding of internal control over financial reporting, but not
for the purpose of expressing an opinion on the effectiveness of
the Company’s internal control over financial reporting.
Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of
material misstatement of the financial statements, whether due to
error or fraud, and performing procedures that respond to those
risks. Such procedures included examining, on a test basis,
evidence regarding the amounts and disclosures in the financial
statements. Our audits also included evaluating the accounting
principles used and significant estimates made by management, as
well as evaluating the overall presentation of the financial
statements. We believe that our audits provide a reasonable basis
for our opinion.
The accompanying consolidated financial statements have been
prepared assuming the Company will continue as a going concern. As
discussed in Note 1 to the
financial statements, the Company has incurred significant
recurring losses. The realization of a major portion of its assets
is dependent upon its ability to meet its future financing needs
and the success of its future operations. These factors raise
substantial doubt about the Company’s ability to continue as
a going concern. The consolidated financial statements do not
include any adjustments that might result from this
uncertainty.
/s/ Seligson &
Giannattasio, LLP
Seligson & Giannattasio, LLP
We have served as the Company’s auditor since
2008.
White Plains, New York
March 27, 2020
GT Biopharma, Inc. and Subsidiaries
Consolidated Balance Sheets
(in thousands, except par value and share data)
|
|
|
|
|
ASSETS
|
|
|
|
Current
Assets:
|
|
|
|
Cash
and cash equivalents
|
$28
|
$60
|
|
Prepaid
expenses
|
246
|
30
|
|
Total
Current Assets
|
274
|
90
|
|
|
|
|
|
Intangible
assets
|
-
|
25,262
|
|
Operating
lease right-to use asset
|
110
|
-
|
|
Deposits
|
12
|
12
|
|
Fixed
assets, net
|
-
|
35
|
|
Total
Other Assets
|
122
|
25,309
|
|
TOTAL
ASSETS
|
$396
|
$25,399
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
|
|
Current
Liabilities:
|
|
|
|
Accounts
payable
|
$1,940
|
$1,762
|
|
Accrued
expenses
|
2,379
|
1,023
|
|
Accrued
interest
|
2,029
|
432
|
|
Line
of credit
|
31
|
31
|
|
Note
Payable to Related Party
|
-
|
100
|
|
Deferred
Rent
|
-
|
8
|
|
Operating
lease liability
|
120
|
|
|
Convertible
debentures
|
13,207
|
10,673
|
|
Total
Current Liabilities
|
19,706
|
14,029
|
|
|
|
|
|
Total
liabilities
|
19,706
|
14,029
|
|
|
|
|
|
Commitments and
Contingencies
|
|
|
|
Stockholders’
(deficit) Equity:
|
|
|
|
Convertible
preferred stock - $0.001 par value; 15,000,000 shares
authorized:
|
|
|
|
Series
C - 96,230 and 96,230 shares issued and outstanding at December 31,
2019 and December 31, 2018, respectively
|
1
|
1
|
|
Series
J – 2,353,548 and 1,163,548 shares issued and outstanding at
December 31, 2019 and December 31, 2018, respectively
|
2
|
1
|
|
Common
stock - $0.001 par value; 750,000,000 shares authorized; and
69,784,699 and 50,650,478 shares issued and outstanding at December
31, 2019 and December 31, 2018, respectively
|
70
|
51
|
|
Additional
paid-in capital
|
548,118
|
540,171
|
|
Accumulated
deficit
|
(567,332)
|
(528,685)
|
|
Noncontrolling
interest
|
(169)
|
(169)
|
|
Total
Stockholders’ (deficit) Equity
|
(19,310)
|
11,370
|
|
TOTAL
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
$396
|
$25,399
|
The accompanying condensed notes are an integral part of these
consolidated financial statements.
GT Biopharma, Inc. and Subsidiaries
Consolidated Statements of Operations
(in thousands except per share data)
|
|
|
|
|
|
|
|
Operating expenses:
|
|
|
|
Research
and development
|
$1,667
|
$9,067
|
|
Selling,
general and administrative expenses
|
9,790
|
12,487
|
|
Loss
on impairment
|
4,599
|
228,515
|
|
Total
operating expenses
|
16,056
|
250,069
|
|
Loss
from operations
|
(16,056)
|
(250,069)
|
|
Other income (expense):
|
|
|
|
Loss
on disposal of assets
|
(20,463)
|
-
|
|
Interest
expense
|
(2,128)
|
(9,117)
|
|
Total
other income (expense)
|
(22,591)
|
(9,117)
|
|
Loss
before provision for income taxes
|
(38,647)
|
(259,186)
|
|
Provision
for income tax
|
-
|
-
|
|
Net
loss
|
$(38,647)
|
$(259,186)
|
|
Net
loss per common share – basic and diluted
|
$(0.67)
|
$(5.16)
|
|
Weighted
average common shares outstanding – basic and
diluted
|
57,527
|
50,240
|
The
accompanying condensed notes are an integral part of these
consolidated financial statements.
GT Biopharma, Inc. and Subsidiaries
Consolidated Statement of Stockholders' Equity
For the Years Ended December 31, 2019 and 2018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance
at December 31, 2018
|
1,260
|
$2
|
50,118
|
$50
|
$521,305
|
$(269,499)
|
|
Issuance of
warrants
|
|
|
|
|
8,304
|
|
|
Issuance of common
stock for convertible notes
|
|
|
162
|
0
|
325
|
|
|
Beneficial conversion
feature on convertible notes
|
|
|
|
|
544
|
|
|
Issuance of common
stock for compensation
|
|
|
370
|
1
|
9,693
|
|
|
Net
loss
|
|
|
|
|
|
(259,186)
|
|
Balance
at December 31, 2018
|
1,260
|
$2
|
50,650
|
$51
|
$540,171
|
$(528,685)
|
|
Issuance of preferred
stock
|
1,190
|
1
|
|
|
1,139
|
|
|
Issuance of common
stock for convertible notes
|
|
|
3,484
|
3
|
1,357
|
|
|
Beneficial conversion
feature on convertible notes
|
|
|
|
|
158
|
|
|
Issuance of common
stock for compensation
|
|
|
15,650
|
16
|
5,293
|
|
|
Net
loss
|
|
|
|
|
|
(38,647)
|
|
Balance
at December 31, 2019
|
2,450
|
$3
|
69,784
|
$70
|
$548,118
|
$(567,332)
|
The
accompanying condensed notes are an integral part of these
consolidated financial statements.
GT Biopharma, Inc. and Subsidiaries
Consolidated Statements of Cash Flows
(In thousands)
|
|
Twelve Months Ended
December 31,
|
|
|
|
|
|
|
|
|
|
CASH
FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
Net
loss
|
$(38,647)
|
$(259,186)
|
|
Adjustments
to reconcile net loss to net cash used in operating
activities:
|
|
|
|
Depreciation
|
4
|
7
|
|
Loss
on impairment of long-lived assets
|
4,599
|
228,515
|
|
Loss
on the disposal of assets
|
20,494
|
-
|
|
Stock
compensation expense for options and warrants issued to
employees and non-employees
|
5,308
|
9,696
|
|
Amortization
of debt discounts
|
505
|
8,663
|
|
Non-cash
interest expense
|
1,140
|
441
|
|
Amortization
of loan costs
|
-
|
1,076
|
|
Changes
in operating assets and liabilities:
|
|
|
|
Prepaid
Expenses
|
(216)
|
(30)
|
|
Other
assets
|
-
|
(3)
|
|
Other
liabilities
|
-
|
8
|
|
Accounts
payable and accrued liabilities
|
3,154
|
136
|
|
Net
cash used in operating activities
|
(3,659)
|
(10,677)
|
|
CASH
FLOWS FROM INVESTING ACTIVITIES:
|
|
|
|
Acquisition
of fixed assets
|
|
(36)
|
|
Disposal
of fixed assets
|
200
|
-
|
|
Net
cash used by investing activities
|
200
|
(36)
|
|
CASH
FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
Proceeds
from notes payable
|
3,527
|
15,145
|
|
Loan
costs
|
-
|
(533)
|
|
Repayment
of note payable
|
(100)
|
(4,415)
|
|
Net
cash provided by financing activities
|
3,427
|
10,197
|
|
NET
(DECREASE) INCREASE IN CASH AND CASH EQUIVALENTS
|
(32)
|
(516)
|
|
CASH
AND CASH EQUIVALENTS - Beginning of period
|
60
|
576
|
|
CASH
AND CASH EQUIVALENTS - End of period
|
$28
|
$60
|
|
|
|
|
|
Supplemental cash flow disclosures:
|
|
|
|
Issuance
of common stock upon conversion of convertible notes
|
$1,360
|
$325
|
|
Issuance
of common stock for interest expense
|
$21
|
$-
|
The
accompanying condensed notes are an integral part of these
consolidated financial statements.
1. The
Company
Business
In 1965, the corporate predecessor of GT Biopharma, Diagnostic
Data, Inc. was incorporated in the State of California.
Diagnostic Data changed its
incorporation to the State of Delaware in 1972; and changed its
name to DDI Pharmaceuticals, Inc. in 1985. In 1994, DDI
Pharmaceuticals merged with International BioClinical, Inc. and
Bioxytech S.A. and changed its name to OXIS International, Inc. In
July 2017, the Company changed its name to GT Biopharma,
Inc.
We are a clinical stage biopharmaceutical company focused on the development and
commercialization of novel immuno-oncology products based off our proprietary
Tri-specific Killer Engager
(TriKE™),
Tetra-specific Killer Engager
(TetraKE™) and
bi-specific ligand-directed single-chain fusion protein technology
platforms. Our TriKE and
TetraKE platforms generate proprietary therapeutics designed to
harness and enhance the cancer killing abilities of a
patient’s own natural killer cells, or NK cells. Once bound
to an NK cell, our moieties are designed to enhance the NK cell and
precisely direct it to one or more specifically-targeted proteins
expressed on a specific type of cancer cell or virus infected cell,
ultimately resulting in the targeted cell’s death.
TriKEs and TetraKEs are made up of
recombinant fusion proteins, can be designed to target any number
of tumor antigens on hematologic malignancies, sarcomas or solid
tumors and do not require patient-specific
customization.
Basis of Consolidation
The accompanying consolidated financial statements include the
accounts of GT Biopharma, Inc. and its subsidiaries.
All intercompany balances and
transactions have been eliminated. The Company’s financial
statements are prepared using the accrual method of
accounting.
Going Concern
The Company’s current operations have focused on business
planning, raising capital, establishing an intellectual property
portfolio, hiring, and conducting preclinical studies and clinical
trials. The Company does not have any product candidates approved for sale and has not
generated any revenue from product sales. The Company has sustained operating
losses since inception and expects such losses to continue over the
foreseeable future.
The financial statements of the Company have been prepared on a
going- concern basis, which contemplates the realization of assets
and the satisfaction of liabilities in the normal course of
business. Accordingly, the financial statements do not include any
adjustments that might be necessary should the Company be unable to
continue in existence.
The Company has incurred substantial losses and negative cash flows
from operations since its inception and has an accumulated deficit
of $567 million and cash of $28 thousand as of December 30, 2019.
The Company anticipates incurring additional losses until such
time, if ever, that it can generate significant sales of its
products currently in development.
Substantial additional financing will be needed by the Company to
fund its operations and to commercially develop its
product candidates. These factors
raise substantial doubt about the Company’s ability to
continue as a going concern.
Management is currently evaluating different strategies to obtain
the required funding for future operations. These strategies may
include but are not limited to: public offerings of equity and/or
debt securities, payments from potential strategic research and
development, and licensing and/or marketing arrangements with
pharmaceutical companies. If
the Company is unable to secure adequate additional funding, its
business, operating results, financial condition and cash flows may
be materially and adversely affected.
Use of Estimates
The financial statements and notes are representations of the Company’s
management, which is responsible for their integrity and
objectivity. These accounting policies conform to accounting
principles generally accepted in the United States of America and
have been consistently applied in the preparation of the financial
statements. The preparation of financial statements requires
management to make estimates and assumptions that affect the
reported amounts of assets, liabilities, revenues and expenses and
disclosures of contingent assets and liabilities at the date of the
financial statements. Actual results could differ from those
estimates.
Segment Information
Operating segments are identified as components of an enterprise
about which separate discrete financial information is available
for evaluation by the chief operating decision-maker in making
decisions regarding resource allocation and assessing performance.
To date, the Company has viewed its operations and manages its
business as one segment operating in the United States of
America.
2. Summary
of Significant Accounting Policies
Advertising and promotional fees
Advertising expenses consist primarily of costs incurred in the
design, development, and printing of Company literature and
marketing materials. The Company expenses all advertising expenditures as incurred. There
were no advertising expenses for the years ended December 31, 2019
and 2018, respectively.
Cash and Cash Equivalents
The Company considers all
highly liquid investments with original maturities of three months
or less to be cash equivalents.
Concentrations of Credit Risk
The Company’s cash and cash equivalents, marketable
securities and accounts receivable are monitored for exposure to
concentrations of credit risk. The Company maintains
substantially all of its cash
balances in a limited number of financial institutions. The
balances are each insured by the Federal Deposit Insurance
Corporation up to $250,000. The Company had no balances in excess
of this limit at December 31, 2019.
Stock Based Compensation to Employees
The Company accounts for its stock-based compensation for employees
in accordance with Accounting
Standards Codification (“ASC”) 718. The Company recognizes in the
statement of operations the grant-date fair value of stock options
and other equity-based compensation issued to employees and
non-employees over the related vesting period.
The Company granted no stock options during the years ended
December 31, 2019 and 2018, respectively.
Long-Lived Assets
Our long-lived assets include property, plant and equipment,
capitalized costs of filing patent applications and other
indefinite lived intangible assets. We evaluate our long-lived
assets for impairment, other than indefinite lived intangible
assets, in accordance with ASC 360, whenever events or changes in
circumstances indicate that the carrying amount of such assets may
not be recoverable. Estimates of future cash flows and timing of
events for evaluating long-lived assets for impairment are based
upon management’s judgment. If any of our intangible or
long-lived assets are considered to be impaired, the amount of
impairment to be recognized is the excess of the carrying amount of
the assets over its fair value.
Applicable long-lived assets are amortized or depreciated over the
shorter of their estimated useful lives, the estimated period that
the assets will generate revenue, or the statutory or contractual
term in the case of patents. Estimates of useful lives and periods
of expected revenue generation are reviewed periodically for
appropriateness and are based upon management’s
judgment.
Impairment of Long-Lived Assets
The Company’s long-lived assets currently consist of
indefinite lived intangible assets associated with IPR&D
(“In-Process Research
& Development”) projects and related capitalized patents
acquired in the acquisition of Georgetown Translational
Pharmaceuticals, Inc. as described in Note 3 below. Intangible
assets associated with IPR&D projects are not amortized until
approval by the Food and Drug Administration (FDA) is obtained in a
major market subject to certain specified conditions and management
judgment. The useful life of an amortizing asset generally is
determined by identifying the period in which substantially
all of the cash flows are expected to
be generated.
The Company evaluates indefinite lived intangible assets for
impairment at least annually and whenever impairment indicators are
present in accordance with ASC 350. When necessary, the Company
records an impairment loss for the amount by which the fair value
is less than the carrying value of these assets. The fair value of
intangible assets other than goodwill is typically determined using
the “relief from royalty method”, specifically the
discounted cash flow method utilizing Level 3 fair value inputs. Some of the more
significant estimates and assumptions inherent in this approach
include: the amount and timing of the projected net cash flows,
which includes the expected impact of competitive, legal and/or
regulatory forces on the projections and the impact of
technological risk associated with IPR&D assets, as well as the
selection of a long-term growth rate; the discount rate, which
seeks to reflect the various risks inherent in the projected cash
flows; and the tax rate, which seeks to incorporate the geographic
diversity of the projected cash flows.
The Company performs impairment testing for all other long-lived assets whenever impairment
indicators are present. When necessary, the Company calculates the
undiscounted value of the projected cash flows associated with the
asset, or asset group, and compares this estimated amount to the
carrying amount. If the carrying amount is found to be greater, we
record an impairment loss for the excess of book value over fair
value.
Income Taxes
The Company accounts for income taxes using the asset and liability
approach, whereby deferred income tax assets and liabilities are
recognized for the estimated future tax effects, based on current
enacted tax laws, of temporary differences between financial and
tax reporting for current and prior periods. Deferred tax assets
are reduced, if necessary, by a valuation allowance if the
corresponding future tax benefits may not be realized.
Net Income (Loss) per Share
Basic net income (loss) per share is computed by dividing the net
loss for the period by the weighted average number of common shares
outstanding during the period. Diluted net income (loss) per share
is computed by dividing the net loss for the period by the weighted
average number of common shares outstanding during the period, plus
the potential dilutive effect of common shares issuable upon
exercise or conversion of outstanding stock options and
warrants during the
period.
During 2019, there were three repricings related to the
conversion
price of the convertible debt and the exercise price of the
warrants.
The Company prepared the calculations of the change in value
pursuant to ASU 2017-11, and
determined there was no deemed dividend to include in the
calculation of earnings per share.
The computation of basic and diluted net loss per share for the
years ended December 31, 2019 and 2018 excludes the
common stock equivalents of the
following potentially dilutive securities because their inclusion
would be anti-dilutive:
|
|
|
|
|
|
|
|
Exercise
of common stock warrants
|
9,065,265
|
1,813,053
|
|
Conversion
of preferred stock into common stock
|
11,768,295
|
1,163,659
|
|
Conversion
of convertible debentures into common stock
|
66,136,870
|
5,704,543
|
|
Exercise
of common stock options
|
40
|
1,113
|
|
|
86,970,470
|
8,682,368
|
Patents
Acquired patents are capitalized at their acquisition cost or fair
value. The legal costs, patent registration fees and models and
drawings required for filing patent applications are capitalized if
they relate to commercially viable technologies. Commercially
viable technologies are those technologies that are projected to
generate future positive cash flows in the near term. Legal costs
associated with patent applications that are not determined to be
commercially viable are expensed as incurred. All research and development costs incurred in
developing the patentable idea are expensed as incurred. Legal fees
from the costs incurred in successful defense to the extent of an
evident increase in the value of the patents are
capitalized.
Capitalized costs for pending patents are amortized on a
straight-line basis over the remaining twenty-year legal life of
each patent after the costs have been incurred. Once each patent is
issued, capitalized costs are amortized on a straight-line basis
over the shorter of the patent’s remaining statutory life,
estimated economic life or ten years.
Fixed Assets
Fixed assets are stated at cost. Depreciation is computed on a
straight-line basis over the estimated useful lives of the assets,
which are 3 to 10 years for machinery and equipment and the shorter
of the lease term or estimated economic life for leasehold
improvements.
Fair Value
The carrying amounts reported in the balance sheets for current
liabilities qualify as financial instruments and are a reasonable
estimate of fair value because of the short period of time between
the origination of such instruments and their expected realization
and their current market rate of interest. The three levels are
defined as follows:
●
Level 1 inputs to the valuation methodology are
quoted prices (unadjusted) for identical assets or liabilities in
active markets. The Company’s Level 1 assets include cash
equivalents, primarily institutional money market funds, whose
carrying value represents fair value because of their short-term
maturities of the investments held by these
funds.
●
Level 2 inputs to the valuation methodology
include quoted prices for similar assets and liabilities in active
markets, and inputs that are observable for the asset or liability,
either directly or indirectly, for substantially the full term of
the financial instrument. The Company’s Level 2 liabilities consist of
liabilities arising from the issuance of convertible securities and
in accordance with ASC 815-40. These liabilities are remeasured
each reporting period if required by ASC 815-40. Fair value is
determined using the Black-Scholes valuation model based on observable
market inputs, such as share price data and a discount rate
consistent with that of a government-issued security of a similar
maturity. There were no such liabilities at December 31, 2019
..
●
Level 3 inputs to the valuation methodology are
unobservable and significant to the fair value measurement. The
Company does not have any assets or liabilities measured
using Level 3
inputs.
Research and Development
Research and development costs are expensed as incurred and
reported as research and development expense. Research and
development costs totaled $1.7 million and $9.1 million for the
years ended December 31, 2019 and 2018, respectively. Research and
development costs for the year ended December 31, 2018 included
non-cash compensation of $6.8 million.
Leases
In February 2016, the Financial Accounting Standards Board
(“FASB”)
issued Accounting Standard
Update (“ASU”) No.
2016-02, “Leases.”
This ASU requires all lessees
to be recognized on the balance sheet as right to use assets and
lease liabilities for the rights and obligations created by lease
arrangements with terms greater than 12 months. The Company adopted
the ASU as of January 1, 2019. The effect of the adoption of the
ASU was to increase the other assets and liabilities by
approximately $174,000.
On September 1, 2017, the Company entered into an
Agreement and Plan of Merger whereby it acquired 100% of the
issued and outstanding capital stock of Georgetown Translational
Pharmaceuticals, Inc. (GTP). In exchange for the ownership of GTP,
the Company issued a total of 16,927,878 shares of its
common stock, having a share price of
$15.00 on the date of the transaction, to the three prior owners of
GTP which represented 33% of the issued and outstanding capital
stock of the Company on a fully diluted basis. $253.8 million of
the value of shares issued was allocated to intangible assets
consisting of a portfolio of three CNS development candidates,
which are classified as IPR&D.
For the year ended December 31, 2018, the Company recorded an intangible asset
impairment charge of $228.5 million related to the portfolio of CNS
IPR&D assets within Operating Expenses, which represents the excess
carrying value compared to fair value. The impairment charge was
the result of both internal and external factors. In the 3rd
quarter of 2018, the Company experienced changes in key senior
management, led by the appointment of a new CEO with extensive
experience in oncology drug development. These changes resulted in
the prioritization of immuno-oncology development candidates
relative to CNS development candidates. In conjunction with these
strategic changes, limited internal resources delayed the
development of the CNS IPR&D assets. The limited resources,
changes in senior leadership, and favorable market conditions for
immuno-oncology development candidates have resulted in the Company
choosing to focus on development of its immuno-oncology portfolio.
In light of this shift in market strategy, the Company performed a
commercial assessment and a valuation of the CNS IPR&D assets,
both to assess fair value and support potential future licensing
efforts. The valuation indicated an excess carrying value over the
fair value of these assets, resulting in the impairment charge
noted above.
The fair value of the CNS IPR&D assets was determined using the
discounted cash flow method which utilized significant estimates
and assumptions surrounding the amount and timing of the projected
net cash flows, which includes the probability of
commercialization, the assumption that the assets would be
out-licensed to third-parties for continued development for upfront
licensing fees and downstream royalty payments based on net sales,
and expected impact of competitive, legal and/or regulatory forces
on the projections, as well as the selection of a long-term growth
rate; the discount rate, which seeks to reflect the various risks
inherent in the projected cash flows; and the tax rate, which seeks
to incorporate the geographic diversity of the projected cash
flows.
On September 19, 2019, the Company entered into an Asset
Purchase Agreement (the
“Agreement”),
pursuant to which the Company sold its rights, titles and
interests, including associated patents, to the pharmaceutical
product designated by the Company as GTB-004 (the “Product”). Under the Agreement, the Product
was purchased by DAS Therapeutics, Inc. who the Company believes is
well positioned to take over the clinical development of the
Product including obtaining timely approval by the
FDA.
The Company received $200,000 at closing. The Company will also
participate in the future commercial value of the Product by
receiving $6,000,000 upon the achievement of certain sales
objectives. In addition, the Company will receive a royalty equal
to 1.5% of U.S. sales until such time as the last of the patents
associated with the Product expires. The Company reflected a loss
in the year ended December 31, 2019 totaling
$20,463,000.
As a result of the loss reported on the sale of the Product, as
well as the response received on inquiries related to the other two
projects, the Company determined that the remaining value related
to these remaining projects should be fully impaired. During the
year ended December 31, 2019, the Company reported an impairment
charge for these projects totaling $4,599,000.
4. Debt
Convertible Notes
On January 22, 2018, the Company entered into a Securities
Purchase Agreement
(“SPA”) with
fourteen accredited investors (individually, a
“Buyer” and
collectively, the “Buyers”) pursuant to which the Company
agreed to issue to the Buyers
senior convertible notes in an aggregate principal amount of
$7,760,510 (the “Notes”), which Notes shall be convertible
into the Company’s common stock, par value $0.001 per share
(the “Common
Stock”) at a price of $4.58 per share, and five-year warrants
to purchase the Company’s Common Stock representing the right
to acquire an aggregate of approximately 1,694,440 shares of Common
Stock (the “Warrants”).
Pursuant to the terms of SPA the Notes were subject to an original
issue discount of 10% resulting in proceeds to the Company of
$7,055,000 from the transaction.
Upon the purchase of the Notes, the Buyers received Warrants to purchase 1,694,440
shares of Common Stock. Such Warrants are exercisable for (5) years
from the date the shares underlying the Warrants are freely
saleable. The initial Exercise
Price is $4.58. According to the terms of the warrant agreement, the Warrants are subject to certain
adjustments depending upon the price and structure of a subsequent
financing, including a qualified financing with gross proceeds of
at least $20 million, as defined in the agreements.
The issuance of the Notes and Warrants were made in reliance on the
exemption provided by Section 4(a)(2) of the Securities Act of
1933, as amended (the “Securities Act”) for the offer and sale of
securities not involving a public offering, and Regulation D
promulgated under the Securities Act.
Contemporaneously with the execution and delivery of the SPA, the
Company and the Buyers executed
and delivered a Registration Rights Agreement (the
“Registration Rights
Agreement”) pursuant to which the Company has agreed to
provide certain registration rights with respect to the
Registrable Securities under the 1933
Act and the rules and regulations promulgated thereunder, and
applicable state securities laws.
Senior Convertible Debentures
On August 2, 2018, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase
Agreement with the purchasers identified on the signature pages
thereto (individually, a “Purchaser,” and collectively, the
“Purchasers”)
pursuant to which the Company issued to the Purchasers one year 10% Senior Convertible Debentures in an aggregate
principal amount of $5,140,000 (the “Debentures”), which Debentures shall be
convertible into the Company’s common stock, par value $0.001
per share (the “Common
Stock”), at a price of $2 per share. The Company used a
portion of these proceeds to repay $4.4 million of the
notes issued on January 22, 2018.
Additionally, the remaining $3.3 million of the notes issued on January 22, 2018 were converted
into the Debentures at the same terms discussed
above.
On September 7, 2018, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase
Agreement with the purchasers identified on the signature pages
thereto (individually, a “Purchaser,” and collectively, the
“Purchasers”)
pursuant to which the Company has issued to the Purchasers one year 10% Senior Convertible Debentures in an aggregate
principal amount of $2,050,000 (the “Debentures”), which Debentures shall be
convertible into the Company’s common stock, par value $0.001
per share (the “Common
Stock”), at an initial
price of $2 per share.
On September 24, 2018, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase
Agreement with the purchasers identified on the signature pages
thereto (individually, a “Purchaser,” and collectively, the
“Purchasers”)
pursuant to which the Company has issued to the Purchasers one year 10% Senior Convertible Debentures in an aggregate
principal amount of $800,000 (the “Debentures”), which Debentures shall be
convertible into the Company’s common stock, par value $0.001
per share (the “Common
Stock”), at an initial price of $2 per
share.
On February 4, 2019, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the 15 purchasers (individually, a
“Purchaser,” and
collectively, the “Purchasers”),
pursuant to which the Company issued to the Purchasers, on February
4, 2019, Secured Convertible
Notes in an aggregate principal amount of $1,352,224 (the
“Notes”),
consisting of gross proceeds of $1,052,224 and settlement of
existing debt of $300,000, which Notes shall be convertible at any
time after issuance into shares (the “Conversion
Shares”) of the Company’s common stock, par value
$0.001 per share (the “Common Stock”),
at an initial conversion price of $0.60 per share (the
“Conversion
Price”).
The Notes accrue interest at the rate of 10% per annum and mature
on August 2, 2019. Interest on the Notes is payable in cash or, at
a Purchaser’s option, in
shares of Common Stock at the Conversion Price. Upon the occurrence
of an event of default, interest accrues at 18% per annum. The
Notes contain customary default provisions, including provisions
for potential acceleration, and covenants, including negative
covenants regarding additional indebtedness and dividends. The
Conversion Price is subject to adjustment due to certain events,
including stock dividends and stock splits, and is subject to
reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
Contemporaneously with the execution and delivery of the Purchase
Agreement, on February 4, 2019, the Company and certain of its
wholly-owned subsidiaries entered into a Security Agreement (the
“Security
Agreement”) with Alpha
Capital Anstalt, as collateral agent on behalf of the
Purchasers, and with the
Purchasers, pursuant to which
the Purchasers have been
granted a first-priority security interest in substantially
all of the assets of the Company and
such subsidiaries securing (i) an aggregate principal amount of
$1,352,224 of Notes and (ii) an aggregate principal amount of
$9,058,962 of the Company’s 10% Senior Convertible Debentures issued on August 2,
2018, September 7, 2018 and September 24, 2018 held by such
Purchasers.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not effect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 14 days after February 4, 2019, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights Agreement. The Form
S-3 was filed by the Company on February 14, 2019 and became
effective on March 11, 2019.
On May 22, 2019, GT Biopharma, Inc. (the “Company”) entered into a Securities Purchase
Agreement (the “Purchase
Agreement”) with the ten purchasers (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers, on
May 22, 2019, Secured
Convertible Notes in an aggregate principal amount of $1,300,000
(the “Notes”),
which Notes shall be convertible at any time after issuance into
shares (the “Conversion
Shares”) of the Company’s common stock, par value
$0.001 per share (the “Common Stock”), at an initial conversion
price of $0.35 per share (the “Conversion Price”).
The Notes accrue interest at the rate of 10% per annum and mature
on November 22, 2019. Interest on the Notes is payable in cash or,
at a Purchaser’s option,
in shares of Common Stock at the Conversion Price. Upon the
occurrence of an event of default, interest accrues at 18% per
annum. The Notes contain customary default provisions, including
provisions for potential acceleration, and covenants, including
negative covenants regarding additional indebtedness and dividends.
The Conversion Price is subject to adjustment due to certain
events, including stock dividends and stock splits, and is subject
to reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not effect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days after May 22, 2019, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights Agreement. The Form
S-1 was filed by the Company on June 21, 2019 and became effective
on July 12, 2019.
Between July 31 and August 28, 2019, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the eleven purchasers (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers, Secured Convertible Notes in an aggregate
principal amount of $975,000 (the “Notes”), which Notes shall be convertible at
any time after issuance into shares (the “Conversion Shares”) of the Company’s
common stock, par value $0.001 per share (the
“Common Stock”), at
an initial conversion price of $0.20 per share (the
“Conversion
Price”).
The Notes accrue interest at the rate of 10% per annum and mature
between January 31 and February 28, 2020. Interest on the Notes is
payable in cash or, at a Purchaser’s option, in shares of Common
Stock at the Conversion Price. Upon the occurrence of an event of
default, interest accrues at 18% per annum. The Notes contain
customary default provisions, including provisions for potential
acceleration, and covenants, including negative covenants regarding
additional indebtedness and dividends. The Conversion Price is
subject to adjustment due to certain events, including stock
dividends and stock splits, and is subject to reduction in certain
circumstances if the Company issues Common Stock or Common Stock
equivalents at an effective price per share that is lower than the
Conversion Price then in effect. The Company may only prepay the
Notes with the prior written consent of the respective
Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not effect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days, one or more registration statements
on Form S-3 (or, if Form S-3 is not then available to the Company,
such form of registration that is then available to effect a
registration for resale of the subject securities) covering the
resale of all Conversion
Shares, subject to certain penalties set forth in the Registration
Rights Agreement. The Form S-1 was filed by the Company on
September 13, 2019 and became effective in October 2,
2019.
On December 19, 2019, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the one purchaser (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers, on
December 19, 2019, Secured
Convertible Notes in an aggregate principal amount of $200,000 (the
“Notes”), which
Notes shall be convertible at any time after issuance into shares
(the “Conversion
Shares”) of the Company’s common stock, par value
$0.001 per share (the “Common Stock”), at an initial conversion
price of $0.20 per share (the “Conversion Price”).
The Notes accrue interest at the rate of 10% per annum and mature
on August 19, 2020. Interest on the Notes is payable in cash or, at
a Purchaser’s option, in
shares of Common Stock at the Conversion Price. Upon the occurrence
of an event of default, interest accrues at 18% per annum. The
Notes contain customary default provisions, including provisions
for potential acceleration, and covenants, including negative
covenants regarding additional indebtedness and dividends. The
Conversion Price is subject to adjustment due to certain events,
including stock dividends and stock splits, and is subject to
reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not affect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days after December 19, 2019, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights
Agreement.
Financing Agreement
On November 8, 2010, the Company entered into a financing
arrangement with Gemini Pharmaceuticals, Inc., a
product development and manufacturing
partner of the Company, pursuant to which Gemini Pharmaceuticals
made a $250,000 strategic equity investment in the Company and
agreed to make a $750,000 purchase order line of credit facility
available to the Company. The outstanding principal of
all Advances under the Line of Credit will bear interest at the rate of
interest of prime plus 2 percent per annum. There is $31,000 due on
this credit line at December 31, 2019.
5. Accrued
Expenses
Accrued Expenses are comprised of the following:
|
|
|
|
|
Rent
|
52,000
|
-
|
|
License
Fee
|
50,000
|
-
|
|
Research
& Development
|
1,675,000
|
585,000
|
|
Professional
Fees
|
95,000
|
162,000
|
|
Consulting
and Advisory Services
|
161,000
|
161,000
|
|
Board
of Directors Service Costs
|
101,000
|
94,000
|
|
Payroll
and Benefits
|
245,000
|
21,000
|
|
Accrued Expenses
|
2,379,000
|
1,023,000
|
6. Related
Party Transactions
On December 21, 2018, Dr. Raymond Urbanski, Chief Executive Officer
and Chairman of the Board, provided a short-term loan of $100,000
to meet immediate capital needs. The loan matured on January 20,
2019 and carries an interest rate of 5%. The loan was repaid in
January, 2019.
7. Stockholders’
Equity
Common Stock
For the year ended December 31, 2018, the Company issued 162,500
shares of common stock upon
conversion of $325,000 of senior convertible notes.
For the year ended December 31, 2018, the Company issued a total of
245,000 shares of Rule 144 restricted common stock in full settlement of outstanding
legal matters, and 125,000 shares of Rule 144 restricted
common stock in connection with
consulting services.
For the year ended December 31, 2019, the Company issued a total
3,484,222 shares of common
stock upon conversion of $1,361,034 in principal and interest on
senior convertible notes.
For the year ended December 31, 2019, the Company issued
CEO Anthony Cataldo
a total of 7,000,000 and
the
Company’s CFO Steven
Weldon a total of 4,500,000
shares of Rule 144 restricted common stock as compensation, and 4,150,000 shares
of Rule 144 restricted common
stock in connection with consulting services.
Preferred Stock
The 96,230 shares of Series C
preferred stock are convertible into 111 shares of the
Company’s common stock at
the option of the holders at any time. The conversion ratio is
based on the average closing bid price of the common stock for the fifteen consecutive trading
days ending on the date immediately preceding the date notice of
conversion is given, but cannot be less than .20 or more than .2889
common shares for each Series C
preferred share. The conversion ratio may be adjusted under certain
circumstances such as stock splits or stock dividends. The Company
has the right to automatically convert the Series C preferred stock into common stock if the Company lists its shares
of common stock on the
Nasdaq National Market and the average
closing bid price of the Company’s common stock on the Nasdaq National Market for 15 consecutive trading
days exceeds $3,000.00. Each share of Series C preferred stock is entitled to the number
of votes equal to .26 divided by the average closing bid price of
the Company’s common
stock during the fifteen consecutive trading days immediately prior
to the date such shares of Series C preferred stock were purchased. In the
event of liquidation, the holders of the Series C preferred stock shall participate on an
equal basis with the holders of the common stock (as if the Series C preferred stock had converted into
common stock) in any distribution of
any of the assets or surplus funds of the Company. The holders
of Series C preferred stock are
entitled to noncumulative dividends if and when declared by the
Company’s board of
directors. No dividends to Series C preferred stockholders were issued or
unpaid through December 31, 2019 .
On September 1, 2017, the Company designated 2,000,000 shares of
Series J Preferred Stock. Shares of Series J Preferred Stock will
have the same voting rights as shares of common stock with each share of Series J Preferred
Stock entitled to one vote at a meeting of the shareholders of
the Corporation. Shares of
Series J Preferred Stock will not be entitled to receive any
dividends, unless and until specifically declared by our
board of directors. The holders of the
Series J Preferred Stock will participate, on an
as-if-converted-to-common stock
basis, in any dividends to the holders of common stock. Each share of the Series J Preferred
Stock is convertible into one share of our common stock at any time at the option of the
holder.
On the same day, the Board issued 1,513, 548 of those shares in
exchange for the cancellation of debt. In the first quarter of
2019, it was discovered that a certificate of designation with
respect to the Series J Preferred Stock had never been filed with
the Office of the Secretary of
State for the State of Delaware. Legal research determined
that despite the fact the Company had issued shares of Series J
Preferred Stock, those shares had, in fact, never
existed.
To remedy the situation, on April 4, 2019, the Company filed a
certificate of designation with the Office of the Secretary State for the State of Delaware
designating a series of preferred stock as Series J-1 Preferred Stock. On April 19, 2019, the
Company issued 2,353,548 of those shares. The issuance was in lieu
of the preferred stock that should have been issued on September 1,
2017, and in settlement for not receiving preferred stock until 20
months after the debt for which the stock was issued was cancelled.
The Company reflected an expense in general and administrative
costs in the year ended December 31, 2019 totaling
$1,140,000.
The Shares are convertible into
shares of common stock of
the Registrant at the rate of
$0.60 per share. The issuance was exempt from the registration
requirements of Section 5 of the Securities Act of 1933 pursuant to
Section 4(2) of the same Act
since the issuance of the Shares did not involve any public offering.
Common Stock Warrants
Warrant transactions for the years ended December 31, 2019 and 2018
are as follows:
|
|
|
Weighted-Average
Exercise Price
|
|
Outstanding,
December 31, 2018
|
-
|
-
|
|
Granted
|
1,813,053
|
0.20
|
|
Exercised
|
-
|
-
|
|
Expired
|
-
|
-
|
|
Outstanding,
December 31, 2019
|
1,813,053
|
-
|
|
Granted
|
-
|
-
|
|
Exercised
|
-
|
|
|
Expired
|
-
|
|
|
Outstanding,
December 31, 2019
|
1,813,053
|
0.20
|
|
|
|
|
|
Exercisable
Warrants:
|
|
|
|
December
31, 2019
|
1,813,053
|
0.20
|
|
December
31, 2018
|
1,813,053
|
0.20
|
Stock Options
The Company reserved 1,333 shares of its common stock at December 31, 2014 for issuance
under the 2014 Stock Incentive
Plan (the “2014 Plan”). The 2014 Plan, approval by
stockholders in May 2015, permits the Company to grant stock
options to acquire shares of the Company’s
common stock, award stock bonuses of
the Company’s common
stock, and grant stock appreciation rights. At December 31, 2019,
87 shares of common stock were
available for grant and options to purchase 40 shares of
common stock are outstanding under the
2014 Plan.
The following table summarizes stock option transactions for the
years ended December 31, 2019 and 2018:
|
|
|
Weighted-Average
Exercise Price
|
|
Outstanding,
December 31, 2017
|
1,246
|
1,320.00
|
|
Granted
|
-
|
-
|
|
Exercised
|
-
|
-
|
|
Expired
|
(133)
|
1,020.00
|
|
Outstanding,
December 31, 2018
|
1,113
|
1,320.00
|
|
Granted
|
-
|
-
|
|
Exercised
|
-
|
-
|
|
Expired
|
(1,073)
|
1,500.00
|
|
Outstanding,
December 31, 2019
|
40
|
877.50
|
|
|
|
|
|
Exercisable
Options:
|
|
|
|
December
31, 2019
|
40
|
877.50
|
|
December
31, 2018
|
1,113
|
1,320.00
|
The following table summarizes information about
all outstanding and exercisable stock
options at December 31, 2019 :
|
|
|
|
|
|
|
Weighted-Average Remaining
Contractual Life
|
Weighted-Average Exercise Price
|
|
Weighted-Average Exercise Price
|
|
$750.00
to$2,225.00
|
40
|
0.89
|
$877.50
|
40
|
$877.50
|
|
|
|
|
|
|
|
8. Income
Taxes
Deferred Taxes
Deferred taxes reflect the net tax effects of temporary differences
between the carrying amounts of assets and liabilities for
financial reporting purposes and the amounts used for income tax
purposes, and operating losses and tax credit carryforwards. The
significant components of net deferred income tax assets for the
Company are (in thousands):
|
|
|
|
|
|
|
|
Deferred
tax assets:
|
|
|
|
Federal
net operating loss carryforward
|
36,803,000
|
25,306,000
|
|
Intellectual
property
|
58,504,000
|
61,787,000
|
|
Accrued
expense
|
1,262,000
|
129,000
|
|
Patent
amortization
|
4,000
|
5,000
|
|
Deferred
tax asseets before valuation
|
96,573,000
|
87,227,000
|
|
Valuation
allowance
|
(96,573,000)
|
(87,227,000)
|
|
Net
deferred income tax assets
|
-
|
-
|
Generally accepted accounting principles requires that the tax
benefit of net operating losses, temporary differences and credit
carryforwards be recorded as an asset to the extent that management
assesses that realization is “more likely than not.”
Realization of the future tax benefits is dependent on the
Company’s ability to generate sufficient taxable income
within the carryforward period. Because of the Company’s
history of operating losses, management has provided a valuation
allowance equal to its net deferred tax assets. The valuation
allowance increased by approximately $9,346,000 during the year
ended December 31, 2019.
Tax Carryforward
At December 31, 2019, the Company had net operating loss
carryforwards of approximately $122,676,000 to reduce United States
federal taxable income in future years. These carryforwards expire
from 2020 through 2039.
The Company is no longer subject to U.S. and state tax examinations
for years ending before the fiscal year ended December 31, 2015.
Management does not believe there will be any material changes in
our unrecognized tax positions over the next twelve
months.
The Company’s policy is to recognize interest and penalties
accrued on any unrecognized tax benefits as a component of income
tax expense. There was no accrued interest or penalties associated
with any unrecognized tax benefits, nor was any interest expense
recognized during the years ended December 31, 2019 and
2018.
9.
Commitments and Contingencies
Leases
On September 1, 2017, the Company entered into a three-year
lease agreement for its office
in Washington, D.C. In addition to minimum rent, certain leases
require payment of real estate taxes, insurance, common area
maintenance charges and other executory costs. The Company
recognizes rent expense under such arrangements on a straight-line
basis over the effective term of each lease. This lease was
terminated as of June 30, 2018.
On October 1, 2018, the Company entered into a three-year
lease agreement for its office
in Westlake Village, CA. In addition to minimum rent, certain
leases require payment of real estate taxes, insurance, common area
maintenance charges and other executory costs. The Company
recognizes rent expense under such arrangements on a straight-line
basis over the effective term of each lease.
The following table summarizes the Company’s future minimum
lease commitments as of December 31, 2019 (in
thousands):
|
Year
ending December 31:
|
|
2020
|
71,000
|
|
2021
|
61,000
|
|
Total
minimum lease payments
|
132,000
|
Rent expense for the years ended December 31, 2019 and 2018 was
$69,000 and $9,000, respectively.
Employment Agreements
On February 14, 2018, the Company entered into the First Amendment
to the Employment Agreement with Dr. Clarence-Smith, amending the
Employment Agreement, dated September 1, 2017, between the Company
and Dr. Clarence-Smith. Under
the First Amendment, Dr.
Clarence-Smith’s title was revised to reflect her new
position and included an annual salary of $500,000, paid in equal
monthly installments. All other
terms of her original Employment Agreement remain unchanged. In
October 2018, Dr.
Clarence-Smith resigned from her position with the Company. In
connection with this resignation, the Company entered into a
separation agreement which
superseded the Employment Agreement.
On October 18, 2018, the Company entered into a Consultant
Agreement with Anthony Cataldo. The term of the Consultant
Agreement shall remain in effect until September 30, 2019. This
Agreement supersedes the Consultant Agreement dated February 14,
2018 and will pay Mr. Cataldo $25,000 per month during the term of
the Agreement.
On October 19, 2018, the Company entered into an Executive
Employment Agreement with Dr. Raymond Urbanski, reflecting his
current position as Chief Executive Officer of the Company. Under
the terms of this agreement,
Dr. Urbanski’s annual salary is essentially unchanged from
his previous positions. Dr. Urbanski is also entitled to
participate in the Company’s bonus plans. Under the Executive
Employment Agreement, the Company has agreed that upon shareholder
approval of a Stock Option
Plan, it will recommend to the Board that the Company grant Dr.
Urbanski a Non-Qualified stock
option to purchase 2,971,102 shares of the Company’s
common stock having an exercise equal
to the fair market value of the shares on the date of the
Agreement. The stock option grant would vest according to the
following schedule: (i) 1,250,000 fully vested shares upon signing
of the agreement, (ii)
1,250,000 shares on January 1, 2019 and (iii) 471,102 shares on
January 1, 2020. On March 15, 2019, Dr, Urbanski resigned his
position as Chief Executive Officer, President and Chairman of the
Board.
10. Subsequent
Events
Financing
On January 30, 2020 GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the one purchaser (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers,
between April 20 and May 7, 2020, Secured Convertible Notes in an aggregate
principal amount of $200,000 (the “Notes”), which Notes shall be convertible at
any time after issuance into shares (the “Conversion Shares”) of the Company’s
common stock, par value $0.001 per share (the
“Common Stock”), at
an initial conversion price of $0.20 per share (the
“Conversion
Price”).
The Notes accrue interest at the rate of 10% per annum and mature
on September 30, 2020. Interest on the Notes is payable in cash or,
at a Purchaser’s option,
in shares of Common Stock at the Conversion Price. Upon the
occurrence of an event of default, interest accrues at 18% per
annum. The Notes contain customary default provisions, including
provisions for potential acceleration, and covenants, including
negative covenants regarding additional indebtedness and dividends.
The Conversion Price is subject to adjustment due to certain
events, including stock dividends and stock splits, and is subject
to reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not effect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days after January 30, 2020, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights
Agreement.
On March 24, 2020 GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the one purchaser (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers, on
January 30, 2020, Secured
Convertible Notes in an aggregate principal amount of $200,000 (the
“Notes”), which
Notes shall be convertible at any time after issuance into shares
(the “Conversion
Shares”) of the Company’s common stock, par value
$0.001 per share (the “Common Stock”), at an initial conversion
price of $0.20 per share (the “Conversion Price”).
The Notes accrue interest at the rate of 10% per annum and mature
on September 30, 2020. Interest on the Notes is payable in cash or,
at a Purchaser’s option,
in shares of Common Stock at the Conversion Price. Upon the
occurrence of an event of default, interest accrues at 18% per
annum. The Notes contain customary default provisions, including
provisions for potential acceleration, and covenants, including
negative covenants regarding additional indebtedness and dividends.
The Conversion Price is subject to adjustment due to certain
events, including stock dividends and stock splits, and is subject
to reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not effect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days after March 24, 2020, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights
Agreement.
Common Stock
In the first quarter of 2020, the Company issued 814,733 shares
of common stock upon conversion
of $162,947 in principal and interest on senior convertible
notes.
GT Biopharma, Inc. and Subsidiaries
as of March 31,2020 and December 31, 2019
(in Thousands, Except Par Value and Share Data)
|
|
|
|
|
ASSETS
|
|
|
|
Current
Assets:
|
|
|
|
Cash
and cash equivalents
|
$5
|
$28
|
|
Prepaid
expenses
|
183
|
246
|
|
Total
Current Assets
|
188
|
274
|
|
|
|
|
|
Deposits
|
12
|
12
|
|
Operating
lease right-to-use asset
|
95
|
110
|
|
Total
Other Assets
|
107
|
122
|
|
TOTAL
ASSETS
|
$295
|
$396
|
|
LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
|
|
|
Current
Liabilities:
|
|
|
|
Accounts
payable
|
$2,370
|
$1,940
|
|
Accrued
expenses
|
2,736
|
2,379
|
|
Accrued
interest
|
2,651
|
2,029
|
|
Operating
lease liability
|
105
|
120
|
|
Line
of credit
|
31
|
31
|
|
Convertible
debentures
|
13,257
|
13,207
|
|
Total
Current Liabilities
|
21,150
|
19,706
|
|
|
|
|
|
Total
liabilities
|
21,150
|
19,706
|
|
|
|
|
|
Commitments
and Contingencies
|
|
|
|
|
|
|
|
Stockholders’
Deficit:
|
|
|
|
Convertible
preferred stock - $0.001 par value; 15,000,000 shares
authorized:
|
|
|
|
Series
C - 96,230 and 96,230 shares issued and outstanding at March 31,
2020 and December 31, 2019, respectively
|
1
|
1
|
|
Series
J – 2,353,548 and 2,353,548 shares issued and outstanding at
March 31, 2020 and December 31, 2019, respectively
|
2
|
2
|
|
Common
stock - $0.001 par value; 750,000,000 shares authorized; and
70,599,433 and 69,784,699 shares issued and outstanding at March
31, 2020 and December 31, 2019, respectively
|
71
|
70
|
|
Additional
paid-in capital
|
548,280
|
548,118
|
|
Accumulated
deficit
|
(569,040)
|
(567,332)
|
|
Noncontrolling
interest
|
(169)
|
(169)
|
Total
Stockholders’ Deficit
|
(20,855)
|
(19,310)
|
|
TOTAL
LIABILITIES AND STOCKHOLDERS’ DEFICIT
|
$295
|
$396
|
The accompanying notes are an integral part of these consolidated
financial statements.
GT Biopharma, Inc. and Subsidiaries
March 31, 2020 and 2019
(in Thousands, Except Par Value and Share Data)
|
|
|
|
|
|
|
|
Revenue:
|
|
|
|
License
revenues
|
$-
|
$-
|
|
TOTAL
REVENUE
|
-
|
-
|
|
Cost
of License Revenue
|
-
|
-
|
|
Gross
profit
|
-
|
-
|
|
Operating
Expenses:
|
|
|
|
Research
and development
|
324
|
834
|
|
Selling,
general and administrative
|
746
|
3,222
|
|
Total
operating expenses
|
1,070
|
4,056
|
|
Loss
from Operations
|
(1,070)
|
(4,056)
|
|
Other
income (expense)
|
|
|
|
Interest
expense/income
|
(638)
|
(454)
|
|
Total
Other Income (Expense)
|
(638)
|
(454)
|
|
Loss
before minority interest and provision for income
taxes
|
(1,708)
|
(4,510)
|
|
Less:
Loss attributable to the noncontrolling interests
|
-
|
-
|
|
Loss
before provision for income taxes
|
(1,708)
|
(4,510)
|
|
Provision
for income taxes
|
-
|
-
|
|
Net
loss
|
(1,708)
|
(4,510)
|
|
Loss
per share
|
|
|
|
Basic
|
$(0.02)
|
$(0.09)
|
|
Diluted
|
$(0.02)
|
$(0.09)
|
|
|
|
|
|
Weighted
Average Shares Outstanding –
|
|
|
|
Basic
|
70,077,026
|
51,092,886
|
|
Diluted
|
70,077,026
|
51,092,886
|
The accompanying notes are an
integral part of these consolidated financial
statements.
GT Biopharma, Inc. and Subsidiaries
Consolidated Statements of Cash
Flows
For the Three Months Ended March 31, 2020 and 2019
(in Thousands)
|
|
|
|
|
|
|
|
|
CASH
FLOWS FROM OPERATING ACTIVITIES:
|
|
|
|
Net
loss
|
$(1,708)
|
$(4,510)
|
Adjustments to reconcile net loss to net cash used in operating
activities:
|
|
|
Depreciation
|
-
|
1
|
|
Stock
compensation expense for options and warrants issued to
employees and non-employees
|
-
|
2,565
|
|
Amortization
of debt discounts
|
-
|
163
|
|
Changes
in operating assets and liabilities:
|
|
|
|
Other
assets
|
63
|
3
|
|
Accounts
payable and accrued liabilities
|
1,422
|
817
|
|
Net
cash used in operating activities
|
(223)
|
(961)
|
|
CASH
FLOWS FROM FINANCING ACTIVITIES:
|
|
|
|
Proceeds
from notes payable
|
200
|
1,052
|
|
Loan
costs
|
-
|
-
|
|
Repayment
of note payable
|
-
|
(100)
|
|
Net
cash provided by financing activities
|
200
|
952
|
|
Minority
interest
|
-
|
-
|
|
NET
INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS
|
(23)
|
(9)
|
|
CASH
AND CASH EQUIVALENTS - Beginning of period
|
28
|
60
|
|
CASH
AND CASH EQUIVALENTS - End of period
|
$5
|
$51
|
|
|
|
|
|
Supplemental
disclosures:
|
|
|
|
Interest
paid
|
$-
|
$-
|
|
Income
taxes paid
|
$-
|
$-
|
|
|
|
|
|
Supplemental
disclosures:
|
|
|
|
Issuance
of common stock upon conversion of convertible notes
|
$150
|
$430
|
|
Issuance
of common stock upon conversion of accrued interest
|
$12
|
$4
|
The accompanying notes are an integral part of these consolidated
financial statements.
1.
The Company and Summary of Significant Accounting
Policies
Business
In 1965, the corporate predecessor of GT Biopharma, Diagnostic
Data, Inc. was incorporated in the State of California.
Diagnostic Data changed its
incorporation to the State of Delaware in 1972. and changed its
name to DDI Pharmaceuticals, Inc. in 1985. In 1994, DDI
Pharmaceuticals merged with International BioClinical, Inc. and
Bioxytech S.A. and changed its name to OXIS International, Inc. In
July 2017, the Company changed its name to GT Biopharma,
Inc.
We are a clinical stage biopharmaceutical company focused on the
development and commercialization of novel immuno-oncology
products
based off our proprietary Tri-specific Killer
Engager (TriKE™),
Tetra-specific Killer
Engager (TetraKE™) and
bi-specific ligand-directed single-chain fusion protein technology
platforms. Our TriKE and TetraKE
platforms generate proprietary therapeutics designed to harness and
enhance the cancer killing abilities of a patient’s own
natural killer cells, or NK cells. Once bound to an NK cell, our
moieties are designed to enhance the NK cell, and precisely direct
it to one or more specifically-targeted proteins expressed on a
specific type of cancer cell or virus infected cell, ultimately
resulting in the targeted cell’s death. TriKEs and TetraKEs are
made up of recombinant fusion proteins, can be designed to target
any number of tumor antigens on hematologic malignancies, sarcomas
or solid tumors and do not require patient-specific
customization.
Going Concern
The Company’s current operations have focused on business
planning, raising capital, establishing an intellectual property
portfolio, hiring, and conducting preclinical studies and clinical
trials. The Company does not have any product candidates approved for sale and has not
generated any revenue from product sales. The Company has sustained operating
losses since inception and expects such losses to continue over the
foreseeable future.
The financial statements of the Company have been prepared on a
goingconcern basis, which contemplates the realization of
assets and the satisfaction of liabilities in the normal course of
business. Accordingly, the financial statements do not include any
adjustments that might be necessary should the Company be unable to
continue in existence.
The Company has incurred substantial losses and negative cash flows
from operations since its inception and has an accumulated deficit
of $569 million and cash of $5 thousand as of March 31, 2020. The
Company anticipates incurring additional losses until such time, if
ever, that it can generate significant sales of its
products currently in development.
Substantial additional financing will be needed by the Company to
fund its operations and to commercially develop its
product candidates. These factors
raise substantial doubt about the Company’s ability to
continue as a going concern.
Management is currently evaluating different strategies to obtain
the required funding for future operations. These strategies may
include but are not limited to: public offerings of equity and/or
debt securities, payments from potential strategic research and
development, and licensing and/or marketing arrangements with
pharmaceutical companies. If
the Company is unable to secure adequate additional funding, its
business, operating results, financial condition and cash flows may
be materially and adversely affected.
Use of Estimates
The financial statements and notes are representations of the Company’s
management, which is responsible for their integrity and
objectivity. These accounting policies conform to accounting
principles generally accepted in the United States of America, and
have been consistently applied in the preparation of the financial
statements. The preparation of financial statements requires
management to make estimates and assumptions that affect the
reported amounts of assets, liabilities revenues and expenses and
disclosures of contingent assets and liabilities at the date of the
financial statements. Actual results could differ from those
estimates.
Basis of Consolidation and Comprehensive Income
The accompanying consolidated financial statements include the
accounts of GT Biopharma, Inc. and its subsidiaries.
All intercompany balances and
transactions have been eliminated. The Company’s financial
statements are prepared using the accrual method of
accounting.
Basis of Presentation
The accompanying unaudited interim condensed consolidated financial
statements have been prepared in accordance with accounting
principles generally accepted in the U.S. (“U.S. GAAP”)
and the rules and regulations of the U.S. Securities and Exchange
Commission (“SEC”).
Certain information and disclosures required by U.S. GAAP for
complete consolidated financial statements have been condensed or
omitted herein. The interim condensed consolidated financial
statements should be read in conjunction with the audited
consolidated financial statements and notes thereto included in the Company’s Form
10-K for the year ended December 31, 2019 filed with the SEC on
March 27, 2020. The unaudited interim condensed consolidated
financial information presented herein reflects all normal adjustments that are, in the opinion of
management, necessary for a fair statement of the financial
position, results of operations and cash flows for the periods
presented. The Company is responsible for the unaudited interim
consolidated financial statements included in this report. The
results of operations of any interim period are not necessarily
indicative of the results for the full year.
Cash and Cash Equivalents
The Company considers all
highly liquid investments with original maturities of three months
or less to be cash equivalents.
Concentrations of Credit Risk
The Company’s cash and cash equivalents, marketable
securities and accounts receivable are monitored for exposure to
concentrations of credit risk. The Company maintains
substantially all of its cash
balances in a limited number of financial institutions. The
balances are each insured by the Federal Deposit Insurance
Corporation up to $250,000. The Company had no balances in excess
of this limit at March 31, 2020.
Stock Based Compensation to Employees
The Company accounts for its stock-based compensation for employees
in accordance with Accounting
Standards Codification (“ASC”) 718. The Company recognizes
in the statement of operations the grant-date fair value of stock
options and other equity-based compensation issued to employees and
non-employees over the related vesting period.
The Company granted no stock options during the quarters ended
March 31, 2020 and 2019, respectively
Long-Lived Assets
Our long-lived assets include property, plant and equipment,
capitalized costs of filing patent applications and other
indefinite lived intangible assets. We evaluate our long-lived
assets for impairment, other than indefinite lived intangible
assets, in accordance with ASC 360, whenever events or changes in
circumstances indicate that the carrying amount of such assets may
not be recoverable. Estimates of future cash flows and timing of
events for evaluating long-lived assets for impairment are based
upon management’s judgment. If any of our intangible or
long-lived assets are considered to be impaired, the amount of
impairment to be recognized is the excess of the carrying amount of
the assets over its fair value.
Applicable long-lived assets are amortized or depreciated over the
shorter of their estimated useful lives, the estimated period that
the assets will generate revenue, or the statutory or contractual
term in the case of patents. Estimates of useful lives and periods
of expected revenue generation are reviewed periodically for
appropriateness and are based upon management’s
judgment.
Impairment of Long-Lived Assets
The Company evaluates indefinite lived intangible assets for
impairment at least annually and whenever impairment indicators are
present in accordance with ASC 350. When necessary, the Company
records an impairment loss for the amount by which the fair value
is less than the carrying value of these assets. The fair value of
intangible assets other than goodwill is typically determined using
the “relief from royalty method”, specifically the
discounted cash flow method utilizing Level 3 fair value inputs. Some of the more
significant estimates and assumptions inherent in this approach
include: the amount and timing of the projected net cash flows,
which includes the expected impact of competitive, legal and/or
regulatory forces on the projections and the impact of
technological risk associated with IPR&D assets, as well as the
selection of a long-term growth rate; the discount rate, which
seeks to reflect the various risks inherent in the projected cash
flows; and the tax rate, which seeks to incorporate the geographic
diversity of the projected cash flows.
The Company performs impairment testing for all other long-lived assets whenever impairment
indicators are present. When necessary, the Company calculates the
undiscounted value of the projected cash flows associated with the
asset, or asset group, and compares this estimated amount to the
carrying amount. If the carrying amount is found to be greater, we
record an impairment loss for the excess of book value over fair
value.
Income Taxes
The Company accounts for income taxes using the asset and liability
approach, whereby deferred income tax assets and liabilities are
recognized for the estimated future tax effects, based on current
enacted tax laws, of temporary differences between financial and
tax reporting for current and prior periods. Deferred tax assets
are reduced, if necessary, by a valuation allowance if the
corresponding future tax benefits may not be realized.
Net Income (Loss) per Share
Basic net income (loss) per share is computed by dividing the net
loss for the period by the weighted average number of common shares
outstanding during the period. Diluted net income (loss) per share
is computed by dividing the net loss for the period by the weighted
average number of common shares outstanding during the period, plus
the potential dilutive effect of common shares issuable upon
exercise or conversion of outstanding stock options and
warrants during the period. The
weighted average number of potentially dilutive common shares
excluded from the calculation of net income (loss) per share
totaled in 87,120,470 and 22,731,781 as of March 31, 2020 and 2019,
respectively.
Patents
Acquired patents are capitalized at their acquisition cost or fair
value. The legal costs, patent registration fees and models and
drawings required for filing patent applications are capitalized if
they relate to commercially viable technologies. Commercially
viable technologies are those technologies that are projected to
generate future positive cash flows in the near term. Legal costs
associated with patent applications that are not determined to be
commercially viable are expensed as incurred. All research and development costs incurred in
developing the patentable idea are expensed as incurred. Legal fees
from the costs incurred in successful defense to the extent of an
evident increase in the value of the patents are
capitalized.
Capitalized cost for pending patents are amortized on a
straight-line basis over the remaining twenty year legal life of
each patent after the costs have been incurred. Once each patent is
issued, capitalized costs are amortized on a straight-line basis
over the shorter of the patent’s remaining statutory life,
estimated economic life or ten years.
Fixed Assets
Fixed assets is stated at cost. Depreciation is computed on a
straight-line basis over the estimated useful lives of the assets,
which are 3 to 10 years for machinery and equipment and the
shorter of the lease term or estimated economic life for leasehold
improvements.
Fair Value
The carrying amounts reported in the balance sheets for receivables
and current liabilities each qualify as financial instruments and
are a reasonable estimate of fair value because of the short period
of time between the origination of such instruments and their
expected realization and their current market rate of interest. The
three levels are defined as follows:
●
Level 1 inputs to the valuation methodology are
quoted prices (unadjusted) for identical assets or liabilities in
active markets. The Company’s Level 1 assets include cash
equivalents, primarily institutional money market funds, whose
carrying value represents fair value because of their short-term
maturities of the investments held by these
funds.
●
Level 2 inputs to the valuation methodology
include quoted prices for similar assets and liabilities in active
markets, and inputs that are observable for the asset or liability,
either directly or indirectly, for substantially the full term of
the financial instrument. The Company’s Level 2 liabilities consist of
liabilities arising from the issuance of convertible securities and
in accordance with ASC 815-40: a warrant liability for detachable
warrants, as well as an accrued
derivative liability for the beneficial conversion feature. These
liabilities are remeasured each reporting period. Fair value is
determined using the Black-Scholes valuation model based on observable
market inputs, such as share price data and a discount rate
consistent with that of a government-issued security of a similar
maturity. There were not such liabilities at March 31,
2020.
●
Level
3 inputs to the valuation methodology are unobservable and
significant to the fair value measurement. There were no such
assets or liabilities as of March 31, 2020.
Research and Development
Research and development costs are expensed as incurred and
reported as research and development expense. Research and
development costs totaling $.3 million and $.8 million for the
three months ended March 31, 2020 and 2019,
respectively.
Revenue Recognition
License Revenue
License arrangements may consist of non-refundable upfront license
fees, exclusive licensed rights to patented or patent pending
technology, and various performance or sales milestones and
future product royalty
payments. Some of these arrangements are multiple element
arrangements.
Non-refundable, up-front fees that are not contingent on any future
performance by us, and require no consequential continuing
involvement on our part, are recognized as revenue when the license
term commences and the licensed data, technology and/or compound is
delivered. We defer recognition of non-refundable upfront fees if
we have continuing performance obligations without which the
technology, right, product or
service conveyed in conjunction with the non-refundable fee has no
utility to the licensee that is separate and independent of our
performance under the other elements of the arrangement. In
addition, if we have continuing involvement through research and
development services that are required because our know-how and
expertise related to the technology is proprietary to us, or can
only be performed by us, then such up-front fees are deferred and
recognized over the period of continuing
involvement.
Payments related to substantive, performance-based milestones in a
research and development arrangement are recognized as revenue upon
the achievement of the milestones as specified in the
underlying agreements when they
represent the culmination of the earnings process. As of March 31,
2020, the Company has not generated any licensing
revenue.
Convertible Notes
On January 22, 2018, the Company entered into a Securities Purchase
Agreement (“SPA”)
with fourteen accredited investors (individually, a
“Buyer” and
collectively, the “Buyers”) pursuant to which the Company
agreed to issue to the Buyers
senior convertible notes in an aggregate principal amount of
$7,760,510 (the “Notes”), which Notes shall be convertible
into the Company’s common stock, par value $0.001 per share
(the “Common
Stock”) at a price of $4.58 per share, and five-year warrants
to purchase the Company’s Common Stock representing the right
to acquire an aggregate of approximately 1,694,440 shares of Common
Stock (the “Warrants”).
Pursuant to the terms of SPA the Notes were subject to an original
issue discount of 10% resulting in proceeds to the Company of
$7,055,000 from the transaction.
Upon the purchase of the Notes, the Buyers received Warrants to purchase 1,694,440
shares of Common Stock. Such Warrants are exercisable for (5) years
from the date the shares underlying the Warrants are freely
saleable. The initial Exercise
Price is $4.58. According to the terms
of the warrant
agreement,
the Warrants are subject to certain adjustments depending upon the
price and structure of a subsequent financing, including a
qualified financing with gross proceeds of at least $20
million, as defined in
the agreements.
The issuance of the Notes and Warrants were made in reliance on the
exemption provided by Section 4(a)(2) of the Securities Act of
1933, as amended (the “Securities Act”) for the offer and sale of
securities not involving a public offering, and Regulation D
promulgated under the Securities Act.
Contemporaneously with the execution and delivery of the SPA, the
Company and the Buyers executed
and delivered a Registration Rights Agreement (the
“Registration Rights
Agreement”) pursuant to which the Company has agreed to
provide certain registration rights with respect to the
Registrable Securities under the 1933
Act and the rules and regulations promulgated thereunder, and
applicable state securities laws.
Senior Convertible Debentures
On August 2, 2018, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement with the purchasers identified
on the signature pages thereto (individually, a
“Purchaser,” and
collectively, the “Purchasers”) pursuant to which the Company
issued to the Purchasers one
year 10% Senior Convertible
Debentures in an aggregate principal amount of $5,140,000 (the
“Debentures”),
which Debentures shall be convertible into the Company’s
common stock, par value $0.001 per share (the
“Common Stock”), at
a price of $2 per share. The Company used a portion of these
proceeds to repay $4.4 million of the notes issued on January 22, 2018. Additionally,
the remaining $3.3 million of the notes issued on January 22, 2018 were converted
into the Debentures at the same terms discussed
above.
On September 7, 2018, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement with the purchasers identified
on the signature pages thereto (individually, a
“Purchaser,” and
collectively, the “Purchasers”) pursuant to which the Company
has issued to the Purchasers
one year 10% Senior Convertible
Debentures in an aggregate principal amount of $2,050,000 (the
“Debentures”),
which Debentures shall be convertible into the Company’s
common stock, par value $0.001 per share (the
“Common Stock”), at
an initial price of $2 per share.
On September 24, 2018, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement with the purchasers identified
on the signature pages thereto (individually, a
“Purchaser,” and
collectively, the “Purchasers”) pursuant to which the Company
has issued to the Purchasers
one year 10% Senior Convertible
Debentures in an aggregate principal amount of $800,000 (the
“Debentures”),
which Debentures shall be convertible into the Company’s
common stock, par value $0.001 per share (the
“Common Stock”), at
an initial price of $2 per share.
On February 4, 2019, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the purchasers identified on the signature
pages thereto (individually, a “Purchaser,” and collectively, the
“Purchasers”),
pursuant to which the Company issued to the Purchasers, on February 4, 2019,
Secured Convertible Notes in an
aggregate principal amount of $1,352,224 (the
“Notes”),
consisting of gross proceeds of $1,052,224 and settlement of
existing debt of $300,000, which Notes shall be convertible at any
time after issuance into shares (the “Conversion Shares”) of the Company’s
common stock, par value $0.001 per share (the
“Common Stock”), at
an initial conversion price of $0.60 per share (the
“Conversion
Price”).
The Notes accrue interest at the rate of 10% per annum and mature
on August 2, 2019. Interest on the Notes is payable in cash or, at
a Purchaser’s option, in
shares of Common Stock at the Conversion Price. Upon the occurrence
of an event of default, interest accrues at 18% per annum. The
Notes contain customary default provisions, including provisions
for potential acceleration, and covenants, including negative
covenants regarding additional indebtedness and dividends. The
Conversion Price is subject to adjustment due to certain events,
including stock dividends and stock splits, and is subject to
reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
Contemporaneously with the execution and delivery of the Purchase
Agreement, on February 4, 2019, the Company and certain of its
wholly-owned subsidiaries entered into a Security Agreement (the
“Security
Agreement”) with Alpha
Capital Anstalt, as collateral agent on behalf of the
Purchasers, and with the
Purchasers, pursuant to which
the Purchasers have been
granted a first-priority security interest in substantially
all of the assets of the Company and
such subsidiaries securing (i) an aggregate principal amount of
$1,352,224 of Notes and (ii) an aggregate principal amount of
$9,058,962 of the Company’s 10% Senior Convertible Debentures issued on August 2,
2018, September 7, 2018 and September 24, 2018 held by such
Purchasers.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not affect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 14 days after February 4, 2019, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights Agreement. The Form
S-3 was filed by the Company on February 14,
2019.
On May 22, 2019, GT Biopharma, Inc. (the “Company”) entered into a Securities Purchase
Agreement (the “Purchase
Agreement”) with the ten purchasers (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers, on
May 22, 2019, Secured
Convertible Notes in an aggregate principal amount of $1,300,000
(the “Notes”),
which Notes shall be convertible at any time after issuance into
shares (the “Conversion
Shares”) of the Company’s common stock, par value
$0.001 per share (the “Common Stock”), at an initial conversion
price of $0.35 per share (the “Conversion Price”).
The Notes accrue interest at the rate of 10% per annum and
mature on November 22, 2019. Interest on the Notes is payable in
cash or, at a Purchaser’s
option, in shares of Common Stock at the Conversion Price. Upon the
occurrence of an event of default, interest accrues at 18% per
annum. The Notes contain customary default provisions, including
provisions for potential acceleration, and covenants, including
negative covenants regarding additional indebtedness and dividends.
The Conversion Price is subject to adjustment due to certain
events, including stock dividends and stock splits, and is subject
to reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not affect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days after May 22, 2019, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights Agreement. The Form
S-1 was filed by the Company on June 21, 2019 and became effective
on July 12, 2019.
Between July 31 and August 28, 2019, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the eleven purchasers (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers, Secured Convertible Notes in an aggregate
principal amount of $975,000 (the “Notes”), which Notes shall be convertible at
any time after issuance into shares (the “Conversion Shares”) of the Company’s
common stock, par value $0.001 per share (the
“Common Stock”), at
an initial conversion price of $0.20 per share (the
“Conversion
Price”).
The Notes accrue interest at the rate of 10% per annum and mature
between January 31 and February 28, 2020. Interest on the Notes is
payable in cash or, at a Purchaser’s option, in shares of Common
Stock at the Conversion Price. Upon the occurrence of an event of
default, interest accrues at 18% per annum. The Notes contain
customary default provisions, including provisions for potential
acceleration, and covenants, including negative covenants regarding
additional indebtedness and dividends. The Conversion Price is
subject to adjustment due to certain events, including stock
dividends and stock splits, and is subject to reduction in certain
circumstances if the Company issues Common Stock or Common Stock
equivalents at an effective price per share that is lower than the
Conversion Price then in effect. The Company may only prepay the
Notes with the prior written consent of the respective
Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not affect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days, one or more registration statements
on Form S-3 (or, if Form S-3 is not then available to the Company,
such form of registration that is then available to effect a
registration for resale of the subject securities) covering the
resale of all Conversion
Shares, subject to certain penalties set forth in the Registration
Rights Agreement. The Form S-1 was filed by the Company on
September 13, 2019 and became effective in October 2,
2019.
On December 19, 2019, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the one purchaser (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers, on
December 19, 2019, Secured
Convertible Notes in an aggregate principal amount of $200,000 (the
“Notes”), which
Notes shall be convertible at any time after issuance into shares
(the “Conversion
Shares”) of the Company’s common stock, par value
$0.001 per share (the “Common Stock”), at an initial conversion
price of $0.20 per share (the “Conversion Price”).
The Notes accrue interest at the rate of 10% per annum and mature
on August 19, 2020. Interest on the Notes is payable in cash or, at
a Purchaser’s option, in
shares of Common Stock at the Conversion Price. Upon the occurrence
of an event of default, interest accrues at 18% per annum. The
Notes contain customary default provisions, including provisions
for potential acceleration, and covenants, including negative
covenants regarding additional indebtedness and dividends. The
Conversion Price is subject to adjustment due to certain events,
including stock dividends and stock splits, and is subject to
reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not affect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days after December 19, 2019, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights
Agreement.
On January 30, 2020 GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with the one purchaser (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers, on
January 30, 2020, Secured
Convertible Notes in an aggregate principal amount of $200,000 (the
“Notes”), which
Notes shall be convertible at any time after issuance into shares
(the “Conversion
Shares”) of the Company’s common stock, par value
$0.001 per share (the “Common Stock”), at an initial conversion
price of $0.20 per share (the “Conversion Price”).
The Notes accrue interest at the rate of 10% per annum and mature
on September 30, 2020. Interest on the Notes is payable in cash or,
at a Purchaser’s option,
in shares of Common Stock at the Conversion Price. Upon the
occurrence of an event of default, interest accrues at 18% per
annum. The Notes contain customary default provisions, including
provisions for potential acceleration, and covenants, including
negative covenants regarding additional indebtedness and dividends.
The Conversion Price is subject to adjustment due to certain
events, including stock dividends and stock splits, and is subject
to reduction in certain circumstances if the Company issues Common
Stock or Common Stock equivalents at an effective price per share
that is lower than the Conversion Price then in effect. The Company
may only prepay the Notes with the prior written consent of the
respective Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not affect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days after January 30, 2020, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights
Agreement.
Financing Agreement
On November 8, 2010, the Company entered into a financing
arrangement with Gemini Pharmaceuticals, Inc., a
product development and manufacturing
partner of the Company, pursuant to which Gemini Pharmaceuticals
made a $250,000 strategic equity investment in the Company and
agreed to make a $750,000 purchase order line of credit facility
available to the Company. The outstanding principal of
all Advances under the Line of Credit will bear interest at the rate of
interest of prime plus 2 percent per annum. There is $31,000 due on
this credit line at March 31, 2020.
Common Stock
In the first quarter of 2020, the Company issued 814,734 shares
of common stock upon conversion
of $162,943 in principal and interest on senior convertible
notes.
Preferred Stock
The 96,230 shares of Series C
preferred stock are convertible into 111 shares of the
Company’s common stock at
the option of the holders at any time. The conversion ratio is
based on the average closing bid price of the common stock for the fifteen consecutive trading
days ending on the date immediately preceding the date notice of
conversion is given, but cannot be less than .20 or more than .2889
common shares for each Series C
preferred share. The conversion ratio may be adjusted under certain
circumstances such as stock splits or stock dividends. The Company
has the right to automatically convert the Series C preferred stock into common stock if the Company lists its shares
of common stock on the
Nasdaq National Market and the average
closing bid price of the Company’s common stock on the Nasdaq National Market for 15 consecutive trading
days exceeds $3,000.00. Each share of Series C preferred stock is entitled to the number
of votes equal to .26 divided by the average closing bid price of
the Company’s common
stock during the fifteen consecutive trading days immediately prior
to the date such shares of Series C preferred stock were purchased. In the
event of liquidation, the holders of the Series C preferred stock shall participate on an
equal basis with the holders of the common stock (as if the Series C preferred stock had converted into
common stock) in any distribution of
any of the assets or surplus funds of the Company. The holders
of Series C preferred stock are
entitled to noncumulative dividends if and when declared by the
Company’s board of
directors. No dividends to Series C preferred stockholders were issued or
unpaid through March 31, 2020.
On September 1, 2017, the Company designated 2,000,000 shares of
Series J Preferred Stock. Shares of Series J Preferred Stock will
have the same voting rights as shares of common stock with each share of Series J Preferred
Stock entitled to one vote at a meeting of the shareholders of
the Corporation. Shares of
Series J Preferred Stock will not be entitled to receive any
dividends, unless and until specifically declared by our
board of directors. The holders of the
Series J Preferred Stock will participate, on an
as-if-converted-to-common stock
basis, in any dividends to the holders of common stock. Each share of the Series J Preferred
Stock is convertible into one share of our common stock at any time at the option of the
holder.
On the same day, the Board issued 1,513, 548 of those shares in
exchange for the cancellation of debt. In the first quarter of
2019, it was discovered that a certificate of designation with
respect to the Series J Preferred Stock had never been filed with
the Office of the Secretary of
State for the State of Delaware. Legal research determined that
despite the fact the Company had issued shares of Series J
Preferred Stock, those shares had, in fact, never
existed.
To remedy the situation, on April 4, 2019, the Company filed a
certificate of designation with the Office of the Secretary State for the State of Delaware
designating a series of preferred stock as Series J-1 Preferred Stock. On April 19, 2019, the Company
issued 2,353,548 of those shares. The issuance was in lieu of the
preferred stock that should have been issued on September 1, 2017,
and in settlement for not receiving preferred stock until 20 months
after the debt for which the stock was issued was cancelled. The
Company reflected an expense in general and administrative costs in
the year ended December 31, 2019 totaling
$1,140,000.
The Shares are convertible into
shares of common stock of
the Registrant at the rate of
$0.20 per share. The issuance was exempt from the registration
requirements of Section 5 of the Securities Act of 1933 pursuant to
Section 4(2) of the same Act
since the issuance of the Shares did not involve any public offering.
4.
Stock Options and Warrants
Stock Options
The following table summarizes stock option transactions for the
quarter ended March 31, 2020:
|
|
|
Weighted
Average Exercise Price
|
|
Outstanding,
December 31, 2019
|
40
|
$877.50
|
|
Granted
|
-
|
-
|
|
Exercised
|
-
|
-
|
|
Expired
|
-
|
-
|
|
Outstanding,
March 31, 2020
|
40
|
$877.50
|
|
Exercisable,
March 31, 2020
|
40
|
$877.50
|
Common Stock Warrants
Warrant transactions for the quarter ended March 31, 2020 are as
follows:
|
|
|
Weighted
Average Exercise Price
|
|
Outstanding
at December 31, 2019:
|
1,813,053
|
$0.20
|
|
Granted
|
-
|
|
|
Forfeited
|
-
|
|
|
Exercised
|
-
|
|
|
Outstanding
at March 31, 2020
|
1,813,053
|
$0.20
|
|
Exercisable
at March 31, 2020
|
1,813,053
|
$0.20
|
5.
Commitments and Contingencies
Leases
On October 1, 2018, the Company entered into a three-year
lease agreement for its office
in Westlake Village, CA. In addition to minimum rent, certain
leases require payment of real estate taxes, insurance, common area
maintenance charges and other executory costs. The Company
recognizes rent expense under such arrangements on a straight-line
basis over the effective term of each lease.
The following table summarizes the Company’s future minimum
lease commitments as of March 31, 2020:
|
Year
ending December 31:
|
|
|
2020
|
53,000
|
|
2021
|
61,000
|
|
Total
minimum lease payments
|
$114,000
|
Rent expense for the quarters ended March 31,
2020 and 2019 was $17,200 and $17,000,
respectively.
Convertible Notes
Between April 20 and May 7, 2020, GT Biopharma, Inc. (the
“Company”) entered
into a Securities Purchase Agreement (the
“Purchase
Agreement”) with eight purchasers (individually, a
“Purchaser,” and
collectively, the “Purchasers”), pursuant to which the Company
issued to the Purchasers,
between April 20 and May 7, 2020, Secured Convertible Notes in an aggregate
principal amount of $2,067,000 (the “Notes”), which Notes shall be convertible at
any time after issuance into shares (the “Conversion Shares”) of the Company’s
common stock, par value $0.001 per share (the
“Common Stock”), at
an initial conversion price of $0.20 per share (the
“Conversion
Price”).
The Notes accrue interest at the rate of 10% per annum and mature
between October 20 and November 7, 2020. Interest on the Notes is
payable in cash or, at a Purchaser’s option, in shares of Common
Stock at the Conversion Price. Upon the occurrence of an event of
default, interest accrues at 18% per annum. The Notes contain
customary default provisions, including provisions for potential
acceleration, and covenants, including negative covenants regarding
additional indebtedness and dividends. The Conversion Price is
subject to adjustment due to certain events, including stock
dividends and stock splits, and is subject to reduction in certain
circumstances if the Company issues Common Stock or Common Stock
equivalents at an effective price per share that is lower than the
Conversion Price then in effect. The Company may only prepay the
Notes with the prior written consent of the respective
Purchasers
thereof.
The Purchase Agreement contains customary representations,
warranties and covenants, including covenants, subject to certain
exceptions, that the Company, until the date on which less than 10%
of the Notes are outstanding, shall not effect any
Variable Rate Transaction (as defined
in the Purchase Agreement) and that, for as long as a
Purchaser holds any Notes or
Conversion Shares, the Company shall amend the terms and conditions
of the Purchase Agreement and the transactions contemplated thereby
with respect to such Purchaser
to give such Purchaser the
benefit of any terms or conditions under which the Company agrees
to issue or sell any Common Stock or Common Stock equivalents that
are more favorable to an investor than the terms and conditions
granted to such Purchaser under the Purchase Agreement and the
transactions contemplated thereby.
In addition, the Company entered into a registration rights agreement (the
“Registration Rights
Agreement”) with the Purchasers, pursuant to which the Company has
agreed to file, within 30 days after April 20, 2020, one or more
registration statements on Form S-3 (or, if Form S-3 is not then
available to the Company, such form of registration that is then
available to effect a registration for resale of the subject
securities) covering the resale of all Conversion Shares, subject to certain
penalties set forth in the Registration Rights
Agreement.
Common Stock
In April 2020, the Company issued 250,000 shares of
common stock upon conversion of
$50,000 in principal on convertible notes.
On May 1, 2020, the Company issued 1,086,429 shares of
restricted common stock for
consulting services.
31,924,929 Shares
____________________________
PROSPECTUS
____________________________