UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
| Quarterly report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For
the quarterly period ended
| Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 |
For the transition period from __________ to ____________.
Commission
File Number
(Exact name of registrant as specified in its charter)
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification Number) |
N/A1
(Address of principal executive offices)
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Trading Symbol | Name of exchange on which registered | ||
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Smaller
reporting company | |
| Emerging
growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
As of May 13, 2025, the registrant had shares of common stock outstanding.
1
Effective as of July 1, 2024, the Company
became a fully remote company. We do not maintain a principal executive office. For purposes of compliance with applicable requirements
of the Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended, any stockholder communication required
to be sent to the Company’s principal executive offices may be directed to
GT BIOPHARMA, INC.
FORM 10-Q
For the Three Months Ended March 31, 2025
Table of Contents
| 2 |
GT BIOPHARMA, INC.
Condensed Balance Sheets
| March 31, 2025 | December 31, 2024 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Restricted cash | ||||||||
| Prepaid expenses and other current assets | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Warrant liability | ||||||||
| Total Current Liabilities | ||||||||
| Stockholders’ Deficit | ||||||||
| Convertible Preferred stock, par value $, shares authorized Series C - shares issued and outstanding at March 31, 2025 and December 31, 2024, respectively | ||||||||
| Common stock, par value $, shares authorized, and shares issued and outstanding as of March 31, 2025 and December 31, 2024, respectively | ||||||||
| Additional paid in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total Stockholders’ Deficit | ( | ) | ( | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | $ | ||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| 3 |
GT BIOPHARMA, INC.
Condensed Statements of Operations
| For The Three Months Ended | ||||||||
| March 31, | ||||||||
| 2025 | 2024 | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Revenues | $ | $ | ||||||
| Operating Expenses: | ||||||||
| Research and development | $ | $ | ||||||
| Selling, general and administrative (including $ and $ from stock compensation for the three months ended March 31, 2025 and 2024, respectively) | ||||||||
| Loss from Operations | ( | ) | ( | ) | ||||
| Other Income (Expense) | ||||||||
| Interest income | ||||||||
| Change in fair value of warrant liability | ||||||||
| Gain on settlement of vendor payable | ||||||||
| Other | ||||||||
| Unrealized gain on short-term investments | ( | ) | ||||||
| Total Other Income, Net | ||||||||
| Net Loss | $ | ( | ) | $ | ( | ) | ||
| Net Loss Per Share - Basic and Diluted | $ | ) | $ | ) | ||||
| Weighted average common shares outstanding - basic and diluted | ||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| 4 |
GT BIOPHARMA, INC.
CONDENSED STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
For The Three Months Ended March 31, 2025 and 2024 (Unaudited):
| Preferred Shares | Common Shares | Additional Paid in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Total | ||||||||||||||||||||||
| Balance, December 31, 2024 | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||
| Exercise of warrants for cash and inducement warrants, net | - | |||||||||||||||||||||||||||
| Issuance of prefunded warrant to settle vendor payable | - | - | ||||||||||||||||||||||||||
| Fair value of vested stock options | - | - | ||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance, March 31, 2025 | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||
| Preferred Shares | Common Shares | Additional Paid in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Total | ||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||
| Fair value of vested stock options | - | - | ||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance, March 31, 2024 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| 5 |
GT BIOPHARMA, INC.
Condensed Statements of Cash Flows
| For The Three Months Ended | ||||||||
| March 31, | ||||||||
| 2025 | 2024 | |||||||
| (Unaudited) | (Unaudited) | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock based compensation | ||||||||
| Change in fair value of warrant liability | ( | ) | ( | ) | ||||
| Unrealized gain on short-term investments | ||||||||
| Changes in operating assets and liabilities: | ||||||||
| (Increase) Decrease in prepaid expenses | ( | ) | ||||||
| Increase (Decrease) in accounts payable and accrued expenses | ( | ) | ( | ) | ||||
| Decrease in operating lease right-of-use assets | ( | ) | ||||||
| Net Cash Used in Operating Activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Sale of investments | ||||||||
| Net Cash Provided by Investing Activities | ||||||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Exercise of warrants and issuance of inducement warrants for cash, net | ||||||||
| Net Cash Provided by Financing Activities | ||||||||
| Net Increase (Decrease) in Cash and Cash Equivalents and Restricted Cash | ( | ) | ||||||
| Cash and Cash Equivalents and Restricted Cash at Beginning of Period | ||||||||
| Cash and Cash Equivalents and Restricted Cash at End of Period | $ | $ | ||||||
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | ||||||||
| Cash paid during the period for: | ||||||||
| Interest | $ | $ | ||||||
| Income taxes | $ | $ | ||||||
| SUPPLEMENTAL DISCLOSURES OF NON-CASH INVESTING AND FINANCING ACTIVITIES | ||||||||
| Fair value of prefunded warrant to settle vendor payable | $ | $ | ||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
| 6 |
GT BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Three Months Ended March 31, 2025 and 2024 (Unaudited)
Note 1 – Organization and Going Concern Analysis
Organization
GT Biopharma, Inc. (the “Company”) is a clinical stage biopharmaceutical company focused on the development and commercialization of novel immune-oncology products based on our proprietary Tri-specific Killer Engager (TriKE®), and Tetra-specific Killer Engager (Dual Targeting TriKE®) platforms. The Company’s TriKE® and Dual Targeting TriKE® platforms generate proprietary therapeutics designed to harness and enhance the cancer killing abilities of a patient’s own natural killer cells (NK cells).
The corporate predecessor of GT Biopharma, Inc, Diagnostic Data, Inc., was incorporated in the state of California in 1965. Diagnostic Data, Inc. changed its incorporation to the state of Delaware on December 21, 1972 and changed its name to DDI Pharmaceuticals, Inc. on March 11, 1985. On September 7, 1994, DDI Pharmaceuticals, Inc. merged with International BioClinical, Inc. and Bioxytech S.A. and changed its name to OXIS International, Inc. On July 17, 2017, OXIS International, Inc. changed its name to GT Biopharma, Inc.
Throughout this Quarterly Report on Form 10-Q, the terms “GTBP,” “we,” “us,” “our,” “the Company” and “our Company” refer to GT Biopharma, Inc.
The GT Biopharma logo, TriKE®, and other trademarks or service marks of GT Biopharma, Inc. appearing in this quarterly report are the property of the Company. This quarterly report on Form 10-Q also contains registered marks, trademarks and trade names of other companies. All other trademarks, registered marks and trade names appearing herein are the property of their respective holders.
Going Concern Analysis
The
accompanying unaudited condensed financial statements have been prepared assuming that the Company will continue as a going concern.
The Company does not have any product candidates approved for sale and has not generated any revenue from its product sales. The Company
has sustained operating losses since inception, and expects such losses to continue into the foreseeable future. Historically, the Company
has financed its operations through public and private sales of common stock, issuance of preferred and common stock, issuance of convertible
debt instruments, and strategic collaborations. For the three months ended March 31, 2025, the Company recorded a net loss of approximately
$
The unaudited condensed financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern. Accordingly, the unaudited condensed financial statements have been prepared on a basis that assumes the Company will continue as a going concern and which contemplates the realization of assets and satisfaction of liabilities and commitments in the ordinary course of business.
The Company has evaluated the significance of the uncertainty regarding the Company’s financial condition in relation to its ability to meet its obligations, which has raised substantial doubt about the Company’s ability to continue as a going concern. While it is very difficult to estimate the Company’s future liquidity requirements, the Company believes if it is unable to obtain additional financing, existing cash resources will not be sufficient to enable it to fund the anticipated level of operations through one year from the date the accompanying unaudited condensed financial statements are issued. There can be no assurances that the Company will be able to secure additional financing on acceptable terms. In the event the Company does not secure additional financing, the Company will be forced to delay, reduce, or eliminate some or all of its discretionary spending, which could adversely affect the Company’s business prospects, ability to meet long-term liquidity needs and the ability to continue operations.
| 7 |
Note 2 – Summary of Significant Accounting Policies
Basis of Presentation
The accompanying condensed financial statements are unaudited. These unaudited interim condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and applicable rules and regulations of the Securities and Exchange Commission (“SEC”) regarding interim financial reporting. Certain information and note disclosures normally included in the financial statements prepared in accordance with GAAP have been condensed or omitted pursuant to such rules and regulations. Accordingly, these unaudited interim condensed financial statements should be read in conjunction with the financial statements and notes thereto contained in the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2024 filed with the SEC on February 21, 2025 (the “2024 Annual Report”). The balance sheet as of December 31, 2024 included herein, was derived from the audited financial statements as of that date.
In the opinion of management, the accompanying unaudited condensed financial statements contain all adjustments necessary to fairly present the Company’s financial position and results of operations for the interim periods reflected. Except as noted, all adjustments contained herein are of a normal recurring nature. Results of operations for the fiscal periods presented herein are not necessarily indicative of fiscal year-end results.
Accounting Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include management’s estimates for continued liquidity, accruals for potential liabilities, assumptions used in deriving the fair value of derivative liabilities, valuation of equity instruments issued for debt and services and realization of deferred tax assets.
Cash Equivalents
The
Company considers highly liquid investments with maturities of three months or less at the date of acquisition as cash equivalents in
the accompanying unaudited condensed financial statements. At March 31, 2025 and December 31, 2024, total cash equivalents which consist
of money market funds, amounted to approximately $
Restricted Cash
As of March 31, 2025, the Company has classified certain cash balances as restricted cash in its unaudited condensed balance sheets. The Company’s restricted cash is deposited in a financial institution and held as a collateral for a credit card agreement with the same financial institution.
Fair Value of Financial Instruments
Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820-10 requires entities to disclose the fair value of financial instruments, both assets and liabilities recognized and not recognized on the balance sheet for which it is practicable to estimate fair value. ASC 820-10 defines the fair value of a financial instrument as the amount at which the instrument could be exchanged in a current transaction between willing parties.
The three levels of the fair value hierarchy are as follows:
Level 1 Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that the entity has the ability to access.
Level 2 Valuations based on quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable data for substantially the full term of the assets or liabilities.
Level 3 Valuations based on inputs that are unobservable, supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The carrying amounts of the Company’s other financial assets and liabilities, such as cash and cash equivalents, prepaid expenses and other current assets, accounts payable, and accrued expenses, approximate their fair values because of the short maturity of these instruments.
The
carrying amount of the Company’s warrant liability of $
Warrant Liability
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging” (“ASC 815”). For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations.
| 8 |
The Company’s use of derivative financial instruments is generally limited to warrants issued by the Company that do not meet the criteria for equity treatment and are recorded as liabilities. We do not use financial instruments or derivatives for any trading purposes.
Stock-Based Compensation
The Company periodically issues stock-based compensation to officers, directors, employees, and consultants for services rendered. Such issuances vest and expire according to terms established at the issuance date.
Stock-based payments to officers, directors, employees, and consultants in exchange for goods and services, which include grants of employee stock options, are recognized in the financial statements based on their grant date fair values in accordance with ASC 718, Compensation-Stock Compensation. Stock based payments to officers, directors, employees, and consultants, which are generally time vested, are measured at the grant date fair value and depending on the conditions associated with the vesting of the award, compensation cost is recognized on a straight-line or graded basis over the vesting period. Recognition of compensation expense for non-employees is in the same period and manner as if the Company had paid cash for the services. The fair value of stock options granted is estimated using the Black-Scholes option-pricing model, which uses certain assumptions related to risk-free interest rates, expected volatility, expected life, and future dividends. The assumptions used in the Black-Scholes option pricing model could materially affect compensation expense recorded in future periods.
Research and Development Expenses
Costs incurred for research and development are expensed as incurred. The salaries, benefits, and overhead costs of personnel conducting research and development of the Company’s products are included in research and development expenses. Purchased materials that do not have an alternative future use are also expensed.
Basic earnings (loss) per share is computed using the weighted-average number of common shares outstanding during the period. Diluted earnings (loss) per share is computed using the weighted-average number of common shares and the dilutive effect of contingent shares outstanding during the period. Potentially dilutive contingent shares, which primarily consist of stock issuable upon exercise of stock options and warrants have been excluded from the diluted loss per share calculation because their effect is anti-dilutive.
March 31, 2025 (Unaudited) | March 31, 2024 (Unaudited) | |||||||
| Options to purchase common stock | ||||||||
| Warrants to purchase common stock | ||||||||
| Total anti-dilutive securities | ||||||||
Concentration
Cash
is deposited in one financial institution. The balances held at this financial institution at times may be in excess of Federal Deposit
Insurance Corporation (“FDIC”) insurance limits of up to $
The Company has a significant concentration of expenses incurred from and accounts payable to Cytovance, a related party, and the University of Minnesota, see Note 3 – Accounts Payable and Related Party.
Segment Information
The Company’s Chief Executive Officer and President (“CEO”) is our chief operating decision maker (“CODM”) and evaluates performance and makes operating decisions about allocating resources based on financial data presented on a consolidated basis. Because our CODM evaluates financial performance on a consolidated basis, the Company has determined that it operates as a single reportable segment composed of the financial results of GT Biopharma, Inc.
Recent Accounting Pronouncements
In November 2024, the FASB issued ASU 2024-03, Income Statement-Reporting Comprehensive Income-Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses, requiring public entities to disclose additional information about specific expense categories in the notes to the financial statements on an interim and annual basis. ASU 2024-03 is effective for fiscal years beginning after December 15, 2026, and for interim periods beginning after December 15, 2027, with early adoption permitted. The Company is currently evaluating the impact of adopting ASU 2024-03.
| 9 |
The Company’s management has evaluated all the recently issued, but not yet effective, accounting standards and guidance that have been issued or proposed by the FASB or other standards-setting bodies through the filing date of these financial statements and does not believe the future adoption of any such pronouncements will have a material effect on the Company’s financial position and results of operations.
Note 3 – Accounts Payable and Related Party
Accounts payable consists of the following:
| As of | As of | |||||||||||||||
| March 31, 2025 | % | December 31, 2024 | % | |||||||||||||
| Accounts payable to Cytovance, a related party 1 | $ | % | $ | % | ||||||||||||
| Accounts payable to University of Minnesota | % | % | ||||||||||||||
| Legal services firm | % | % | ||||||||||||||
| Other accounts payable | % | % | ||||||||||||||
| Total accounts payable | $ | % | $ | % | ||||||||||||
| 1 |
The details of the Company’s accounts payable to Cytovance were as follows:
| Three Months Ending | ||||||||
| March 31, 2025 | March 31, 2024 | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Beginning balance | $ | $ | ||||||
| Invoices, net | ||||||||
| Payments in cash | ( | ) | ( | ) | ||||
| Payments in pre-funded warrants, at fair value | ( | ) | ||||||
| Ending balance | $ | $ | ||||||
During
the three months ended March 31, 2025, the Company issued pre-funded warrants to purchase up to
In
March 2025, a legal services firm currently engaged by the Company agreed to reduce the Company’s prior year unpaid fees by approximately
$
University of Minnesota
See Note 7 – Commitments and Contingencies, Significant Agreements.
Note 4 – Warrant Liability
2023 Warrants
On
January 4, 2023, as part of the private placement offering, the Company issued common stock, warrants to purchase up to an aggregate
of
| 10 |
The 2023 Common Warrants and the 2023 Placement Agents Warrants (collectively the “2023 Warrants”), provide for a value calculation for the warrants using the Black Scholes model in the event of certain fundamental transactions. The fair value calculation provides for a floor on the volatility amount utilized in the value calculation at % or greater. The Company has determined this provision introduces leverage to the holders that could result in a value that would be greater than the settlement amount of a fixed-for-fixed option on the Company’s own equity shares. Therefore, pursuant to ASC 815, the Company has classified the 2023 Warrants as a liability in its balance sheet. The classification of the 2023 Warrants, including whether they should be recorded as liability or as equity, is evaluated at the end of each reporting period.
The
2023 Warrants were initially recorded at a fair value at $
The 2023 Warrant liability is valued using a BlackScholes Option pricing model with the following assumptions:
| 2023 Warrants | ||||||||
| March 31, 2025 | December 31, 2024 | |||||||
| (Unaudited) | ||||||||
| Stock price | $ | $ | ||||||
| Risk-free interest rate 1 | % | % | ||||||
| Expected volatility 2 | % | % | ||||||
| Expected life (in years) 3 | ||||||||
| Expected dividend yield 4 | ||||||||
| Fair value of warrants | $ | $ | ||||||
| 1 |
| 2 |
| 3 |
| 4 |
The details of warrant liability transactions were as follows:
| Three Months Ending | ||||||||
| March 31, 2025 | March 31, 2024 | |||||||
| (Unaudited) | (Unaudited) | |||||||
| Beginning balance | $ | $ | ||||||
| Issuance of warrants at fair value | ||||||||
| Change in fair value | ( | ) | ( | ) | ||||
| Extinguishment | ||||||||
| Ending balance | $ | $ | ||||||
Note 5 – Stockholders’ Equity (Deficit)
The Company’s authorized capital as of March 31, 2025 was shares of common stock, par value $ per share, and shares of preferred stock, par value $ per share.
Common Stock
2025 Warrant Inducement Transaction
On
February 26, 2025, the Company received gross proceeds of $
The
Inducement Warrants consist of i) new Series A Inducement Warrants, representing warrants to purchase up to
| 11 |
The Company determined that under ASC 815, the 2025 inducement and placement agent warrants are considered indexed to the Company’s own stock and eligible for an exception from derivative accounting. Accordingly, the fair value of the 2025 inducement and placement agent warrants are classified as equity.
The
Company recognized the aggregate effect of the modification of warrants and grant of inducement warrants of $
Preferred Stock
Series C Preferred Stock
As of March 31, 2025, there were shares of series C preferred stock, par value $ per share (the “Series C Preferred Stock”) issued and outstanding.
As a result of numerous reverse stock-splits in previous years and the agreement terms for adjusting the rights of the related shares, the shares of Series C Preferred Stock are convertible into an infinitesimal amount of common stock, have no voting rights, and in the event of liquidation, the holders of the Series C Preferred Stock would not participate in any distribution of the assets or surplus funds of the Company. The holders of Series C Preferred Stock also are not currently entitled to any dividends if and when declared by the Board. No dividends to holders of the Series C Preferred Stock were declared or unpaid through March 31, 2025.
Note 6 – Common Stock Warrants and Options
Common Stock Warrants
Stock warrant transactions for the three months ended March 31, 2025 were as follows:
| Three Months Ended March 31, 2025 | ||||||||
| Number of | Weighted Average | |||||||
| Warrants | Exercise Price | |||||||
| Warrants outstanding at December 31, 2024 | $ | |||||||
| Granted | ||||||||
| Forfeited/cancelled | ||||||||
| Exercised | ( | ) | ||||||
| Warrants outstanding at March 31, 2025 | $ | |||||||
| Warrants exercisable at March 31, 2025 | $ | |||||||
As of March 31, 2025, all outstanding warrants were fully vested and exercisable and had an aggregate intrinsic value of approximately $ based on the fair value of the Company’s common stock on March 31, 2025.
Warrants outstanding as of March 31, 2025 are exercisable as follows:
| Warrants Outstanding and Exercisable as of March 31, 2025 | ||||||||||||||
Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | |||||||||||
| $ | N/A | $ | ||||||||||||
| 12 |
Common Stock Options
In April 2022 the Company established the 2022 Omnibus Incentive Plan (the “Plan”). The Plan was approved by our Board and stockholders. The purpose of the Plan is to grant stock and options to purchase our common stock, and other incentive awards, to our employees, directors, and key consultants. The maximum number of shares of common stock that may be issued pursuant to awards granted under the Plan is . The shares of our common stock underlying cancelled and forfeited awards issued under the Plan may again become available for grant under the Plan. As of March 31, 2025, there were stock options outstanding and shares of restricted stock granted in prior years under the Plan, which left shares available for grant under the Plan. The following table summarizes stock option transactions for the three months ended March 31, 2025:
| Three Months Ended March 31, 2025 | ||||||||
| Number of | Weighted Average | |||||||
| Options | Exercise Price | |||||||
| Options outstanding at December 31, 2024 | $ | |||||||
| Granted | ||||||||
| Forfeited/cancelled | ||||||||
| Exercised | ||||||||
| Options outstanding at March 31, 2025 | $ | |||||||
| Options exercisable at March 31, 2025 | $ | |||||||
The weighted average remaining contractual life of all options outstanding, and all options vested and exercisable as of March 31, 2025 was years. Furthermore, the aggregate intrinsic value of all options outstanding and all options vested and exercisable as of March 31, 2025 was approximately $ and $, respectively, in each case based on the fair value of the Company’s common stock on March 31, 2025.
The total fair value of options that vested during the three months ended March 31, 2025 and 2024, was $ and $, respectively, and is included in selling, general and administrative expense in the accompanying unaudited condensed statements of operations. As of March 31, 2025, stock options were vested and exercisable and unvested compensation expense amounted to approximately $.
| Stock Options Exercisable as of March 31, 2025 | ||||||||||||||
Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | |||||||||||
| $ | $ | |||||||||||||
Note 7 – Commitments and Contingencies
Litigation
The Company is involved in certain legal proceedings that arise from time to time in the ordinary course of our business. Except for income tax contingencies, we record accruals for contingencies to the extent that our management concludes that the occurrence is probable and that the related amounts of loss can be reasonably estimated. Legal expenses associated with the contingency are expensed as incurred. There is no current or pending litigation of any significance with the exception of the matters identified below that have arisen under, and are being handled in, the normal course of business:
| 13 |
Ohri Matter
On July 22, 2024, the Company filed an AAA Arbitration Demand against Manu Ohri, its former Chief Financial Officer. In the Demand, the Company asserts claims against Mr. Ohri for breach of his fiduciary duties and breach of contract and seeks a declaratory judgment providing that the Company may characterize Mr. Ohri’s termination as “for cause” under his Employment Agreement, and that the Company may revoke the Separation Agreement entered into between the Company and Mr. Ohri prior to the Company learning of Mr. Ohri’s breaches. In addition to the declaratory judgment, the Company seeks damages arising from Mr. Ohri’s violations, and attorneys’ fees and any forum and arbitration fees. On September 3, 2024, Mr. Ohri filed both a general denial of the Company’s claims against him and counterclaims for breach of his Employment Agreement and Separation Agreement. A final hearing is scheduled for June 10th through June 12th, 2025 in Los Angeles, California. At this stage in the proceedings the Company is not able to determine the probability of the outcome of this matter or a range of reasonably expected losses, if any. The Company believes it has recorded an appropriate accrual for the matter.
TWF Global Matter
On May 24, 2023, TWF Global, LLC (“TWF”) filed a Complaint in the California Superior Court for the County of Los Angeles naming the Company as defendant. The Complaint alleges that TWF is the holder of two Convertible Promissory Notes (“Notes”) and that the Company did not deliver shares of common stock due on conversion in February 2021. TWF was seeking per diem liquidated damages based on the terms of alleged Notes. On July 14, 2023, the Company filed a motion to dismiss for improper forum because the terms of the Notes, as alleged, require disputes to be filed in New York state and federal courts. TWF voluntarily dismissed its Complaint before the California Superior Court of Los Angeles without prejudice. The Company subsequently filed a Summons and Complaint for Interpleader against TWF and Z-One LLC before the Supreme Court of the State of New York County of New York, asking the Supreme Court to determine if the Company’s shares of common stock are properly registered to TWF or Z-One LLC, as both of these entities have made conflicting demands for registration of the shares of common stock. On February 5, 2024, the Company filed a motion for entry of default against TWF, seeking an order directing the Company to register the shares of common stock in the name of Z-One and that the Company be released from all associated liability and claims. The Court denied the motion without prejudice and agreed to reconsider the motion without further briefing upon the filing of a supplemental party affidavit. On May 9, 2024, Z-One filed a motion for summary judgement seeking dismissal of the action, representing that Z-One and TWF have settled their dispute over the entitlement to the Company’s shares of common stock and there is no remaining dispute before the Court. On May 21, 2024, the Company filed a supplemental affidavit in support of its motion for entry of default. On November 14, 2024, the Court held a hearing on the parties’ motions, at which the Court found that the motion for entry of default was mooted by the settlement agreement between Z-One and TWF. The Court ordered that the case be dismissed. On February 17, 2025, Z-One, LLC filed a Summons with Notice in the Supreme Court of the State of New York, County of New York. Z-One alleges that it was assigned TWF’s rights under the Notes and seeks enforcement and damages. The Company intends to demand a complaint, and seek dismissal of the case. The Company believes that the claims related to the Notes are without merit, and will continue to vigorously defend against these claims.
Significant Agreements
Cytovance Biologics, Inc., a Related Party
In October 2020, the Company entered into a Master Services Agreement with Cytovance Biologics, Inc. (“Cytovance”), to perform biologic development and manufacturing services, and to produce and test compounds used in the Company’s potential product candidates. The Company subsequently executed numerous Statements of Work (“SOWs”) for the research and development of products for use in clinical trials.
On
August 24, 2022, the Company entered into a Settlement and Investment Agreement with Cytovance that amended existing SOWs and allowed
for future invoices to be settled in in a combination of cash and issuance of the Company’s common stock. The Agreement also set
Cytovance’s beneficial ownership limitation at
On
April 25, 2024, the Company entered into an Amendment to the Settlement and Investment Agreement with Cytovance that increased Cytovance’s
beneficial ownership limitation to
On
June 30, 2024, Cytovance became a related party as their beneficial ownership exceeded
During
the three months ended March 31, 2025 and 2024, the Company recognized research and development expenses of $
As
of March 31, 2025 the Company’s commitments in relation to unbilled and unaccrued SOWs and any related Change Orders from Cytovance
for services that have not yet been rendered as of March 31, 2025, amounted to approximately $
| 14 |
University of Minnesota
2023 Sponsored Research Agreement
On
May 20, 2024, the Company entered into a sponsored research agreement with the Regents of the University of Minnesota (the “2016
Exclusive Patent License Agreement”), effective July 1, 2023, and expiring on July 1, 2025. Payments totaling approximately $
The
Company recorded an expense classified as research and development of approximately $
As
of March 31, 2025 the Company’s commitments in relation to unbilled and unaccrued amounts from the University of Minnesota pursuant
to the 2023 Sponsored Research Agreement for services that have not yet been rendered as of March 31, 2025, amounted to approximately
$
2016 Exclusive Patent License Agreement
Effective
July 18, 2016, the Company entered into an exclusive patent license agreement with the Regents of the University of Minnesota (as amended,
the “2016 Exclusive Patent License Agreement”), to further develop and commercialize cancer therapies using TriKE®
technology developed by researchers at the University of Minnesota to target NK cells to cancer. Under the terms of the agreement,
the Company receives exclusive rights to conduct research and to develop, make, use, sell, and import TriKE® technology
worldwide for the treatment of any disease, state, or condition in humans. The Company is responsible for obtaining all permits, licenses,
authorizations, registrations, and regulatory approvals required or granted by any governmental authority anywhere in the world that
is responsible for the regulation of products such as the TriKE® technology, including without limitation the FDA and
the European Agency for the Evaluation of Medicinal Products in the European Union. The agreement requires an upfront payment of $
Effective
May 13, 2024, the Company entered into an amended and restated exclusive patent license agreement with the Regents of the University
of Minnesota. The amendment requires an upfront payment of $
| 15 |
The Company did not record any expense classified as research and development, pursuant to the 2016 Exclusive Patent License Agreement, for the three months ended March 31, 2025 and 2024, respectively
2021 Exclusive License Agreement
Effective
March 26, 2021, the Company entered into an exclusive license agreement with the Regents of the University of Minnesota (the “2021
Exclusive Patent License Agreement”), specific to the B7H3 targeted TriKE®. The agreement requires an upfront payment
of $
The Company did not record any expense classified as research and development, pursuant to the 2021 Exclusive License Agreement, for the three months ended March 31, 2025 and 2024, respectively.
2024 GTB-3650 Clinical Trial Agreement
On
November 18, 2024, the Registrant entered into an Investigator Initiated Clinical Trial Agreement (the “Agreement”) with
the Regents of the University of Minnesota (the “University”), pursuant to which, the University shall sponsor an Investigational
New Drug (“IND”) application for IND 165546 GTB-3650 (the “Research Program”) and shall serve as a sponsor investigator
for a phase 1 clinical trial entitled, “GTB-3650 (CD16/IL-15/CD33) Tri-Specific Killer Engager (TriKE) for the Treatment of High
Risk Myelodysplastic Syndromes (MDS), Refractory/Relapsed Acute Myeloid Leukemia (AML), and Minimal Residual Disease in AML,” designed
by the University (the “Study”). The Research Program is being conducted for clinical research use. The budget for the Study,
including without limitations, funding and resources, provides for up to approximately $
The Company did not record any expense classified as research and development, pursuant to the 2024 Clinical Trial Agreement, for the three months ended March 31, 2025 and 2024, respectively.
As
of March 31, 2025 the Company’s commitments in relation to unbilled and unaccrued amounts from the University of Minnesota pursuant
to the 2024 Clinical Trial Agreement for services that have not yet been rendered as of March 31, 2025, amounted to approximately $
Contingency – NASDAQ Matters
On
November 21, 2024, we received a letter (the “Notification Letter”) from the Nasdaq Listing Qualifications Staff (the “Staff”)
notifying us that the amount of our stockholders’ equity had fallen below the $
The Notification Letter indicated that we had 45 days (i.e., until January 6, 2025) to submit a plan to regain compliance with the Minimum Stockholders’ Equity Requirement, noting that if such plan is accepted, the Staff can grant us an extension of up to 180 days from the date of the Notification Letter to evidence compliance. In determining whether to accept our plan, the Staff will consider such things as the likelihood that the plan will result in compliance with Nasdaq’s continued listing criteria, our past compliance history, the reasons for our current non-compliance, other corporate events that may occur within the review period, our overall financial condition and our public disclosures.
We submitted a plan of compliance to the Staff on December 31, 2024, outlining our plan to conduct periodic public and private securities offerings to regain compliance. The Staff has not, as yet, accepted our plan of compliance, and has requested additional information regarding our financing plans. We can provide no assurance that the Staff will accept our plan of compliance.
If the Staff does not accept our plan of compliance, or if the Staff accepts our plan but we do not regain compliance within the allotted compliance period, Nasdaq will provide notice that our Common Stock will be subject to delisting. If our Common Stock is delisted from Nasdaq, our ability to raise capital through public offerings of our securities and to finance our operations could be adversely affected. We also believe that delisting would likely result in decreased liquidity and/or increased volatility in our Common Stock and could harm our business and future prospects. In addition, we believe that, if our Common Stock is delisted, our stockholders would likely find it more difficult to obtain accurate quotations as to the price of our Common Stock and it may be more difficult for stockholders to buy or sell our Common Stock at competitive market prices, or at all.
As a result of the private placement
of preferred stock and common stock warrants that occurred on May 12, 2025, the Company believes it has stockholders’ equity on
an unaudited proforma basis of at least $
Note 8 – Segment Information
The Company operates and manages its business as one reportable and operating as a clinical stage biopharmaceutical company focused on the development and commercialization of novel immune-oncology products based on our proprietary Tri-specific Killer Engager (TriKE®), and Tetra-specific Killer Engager (Dual Targeting TriKE®) platforms. The measure of segment assets is reported on the balance sheet as total assets.
The Company’s CODM reviews financial information presented and decides how to allocate resources based on net income (loss). Net income (loss) is used for evaluating financial performance.
Significant segment expenses include research and development, salaries, insurance, and stock-based compensation. Operating expenses include all remaining costs necessary to operate our business, which primarily include external professional services and other administrative expenses. The following table presents the significant segment expenses and other segment items regularly reviewed by our CODM:
| Three Months Ended March 31, | ||||||||
| 2025 | 2024 | |||||||
| Research and development | $ | $ | ||||||
| Salaries | ||||||||
| Insurance | ||||||||
| Stock-based compensation | ||||||||
| Operating expenses | ||||||||
| Other income | ( | ) | ( | ) | ||||
| Net loss | $ | $ | ||||||
| 16 |
Note 9 – Subsequent Events
Private Placement of Preferred Stock and Warrants
On
May 12, 2025, the Company entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with the purchasers
identified therein (collectively, the “Purchasers”) providing for the issuance and sale to the Purchasers of (i) up to
shares of the Company’s Series L 10% Convertible Preferred Stock (the “Preferred Stock”), (ii) warrants to purchase
up to a number of shares of common stock of the Company (the “Common Stock”) equal to 100% of the shares of the Company’s
Common Stock issuable upon conversion of the shares of Preferred Stock (the “Common Warrants”), and (iii) warrants to purchase
up to a number of shares of Company’s Common Stock equal to the number of Greenshoe Conversion Shares (as defined in the Securities
Purchase Agreement) issuable upon exercise of the Greenshoe Right (as defined below) (the “Vesting Warrants” and together
with the Common Warrants, the “Warrants”), with an aggregate stated value of $
Pursuant
to the Securities Purchase Agreement, each Purchaser may elect to purchase shares of Preferred Stock with an aggregate stated value of
up to $
On May 12, 2025, the Company filed a Certificate of Designation of Preferences, Rights and Limitations of Series L 10% Convertible Preferred Stock (the “Certificate of Designation”) with the Secretary of State of the State of Delaware. The Certificate of Designation creates and specifies the rights of Series L 10% Convertible Preferred Stock, including the terms and conditions on which shares of such preferred stock would convert into shares of our Common Stock, as well as its liquidation preference.
Pursuant
to the Certificate of Designation and subject to certain ownership limitations, the Preferred Stock may be converted at any time at the
option of the Purchasers into shares of the Company’s Common Stock at an initial conversion price of $
Pursuant
to the Securities Purchase Agreement, each Purchaser will be issued (i) a Common Warrant, each to purchase up to a number of shares of
the Company’s Common Stock equal to 100% of the Conversion Shares underlying the Preferred Shares issued to such Purchaser and
(ii) a Vesting Warrant (the exercisability of which shall vest ratably from time to time in proportion to the Purchaser’s (or its
permitted assigns’) exercise of such Purchaser’s Greenshoe Rights pursuant to Section 2.4 of the Purchase Agreement), each
to purchase up to a number of shares of the Company’s Common Stock equal to the number of Greenshoe Conversion Shares (as defined
in the Securities Purchase Agreement) applicable to such Purchaser, in accordance with the Securities Purchase Agreement. The Common
Warrants have an initial exercise price of $
The Preferred Shares and Warrants both have full ratchet price protection and are subject to other adjustments, as further described in the Certificate of Designation or the Warrants, as applicable, subject, solely with respect to adjustments in connection with the exercise of Greenshoe Rights, a floor price of $ per share (subject to adjustment for reverse and forward splits, recapitalizations and similar transactions).
The
Company and the Purchasers entered into a registration rights agreement pursuant to which the Company agreed to file a registration statement
with the Securities and Exchange Commission covering the public resale of the Common Stock issuable upon conversion of the Preferred
Stock and upon exercise of the Warrants. The Company has agreed to file a registration statement within 30 days after the initial closing
and after each closing of the exercise of a Greenshoe Right in accordance with the Securities Purchase Agreement, to become effective
no later than 90 days after filing. If these deadlines are not met,
Pursuant to the Securities Purchase Agreement, the Company agreed to hold a meeting of its stockholders at the earliest practical date after the execution of the Securities Purchase Agreement for the purpose of obtaining stockholder approval for the issuance, in the aggregate, of more than % of the number of shares of the Company’s Common Stock outstanding on the date of the initial closing (“Shareholder Approval”). In connection with the required Shareholder Approval, all of the Company’s officers and directors (each a “Voting Agreement Party”) entered into a Voting Agreement pursuant to which each Voting Agreement Party agreed to vote all shares of voting stock over which the Voting Agreement Party has voting control in favor of any resolution presented to the stockholders of the Company seeking Shareholder Approval.
As a result of the private placement of preferred
stock and common stock warrants that occurred on May 12, 2025, the Company believes it has stockholders’ equity on an unaudited
proforma basis of at least $
Private Placement
On May 14, 2025, the Company entered into a common
shares purchase agreement (the “Purchase Agreement”) with investors (collectively, the “Investors”) relating to
a committed equity facility (the “Facility”). Pursuant to the Purchase Agreement, the Company has the right from time to time
at its option to sell to the Investors up to $
Sales of the shares of common stock to the Investors under the Purchase Agreement, and the timing of any sales, will be determined by the Company from time to time in its sole discretion and will depend on a variety of factors, including, among other things, market conditions, the trading price of the common stock and determinations by the Company regarding the use of proceeds of such shares of common stock. The net proceeds from any sales under the Purchase Agreement will depend on the frequency with, and prices at, which the shares of common stock are sold to The Investors.
The purchase price of the shares of common stock that
the Company elects to sell to the Investors pursuant to the Purchase Agreement will be
In connection with the execution of the Purchase
Agreement, the Company agreed to issue to the Investors a pre-funded warrant to purchase
Settlement of Vendor Payable in Stock
Subsequent
to March 31, 2025, the Company issued shares of common stock and warrants to purchase
| 17 |
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements in this Quarterly Report on Form 10-Q are “forward-looking statements” within the meaning of the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding our current beliefs, goals and expectations about matters such as our expected financial position and operating results, our business strategy and our financing plans. The forward-looking statements in this report are not based on historical facts, but rather reflect the current expectations of our management concerning future results and events. The forward-looking statements generally can be identified by the use of terms such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “foresee,” “may,” “guidance,” “estimate,” “potential,” “outlook,” “target,” “forecast,” “likely” or other similar words or phrases. Similarly, statements that describe our objectives, plans or goals are, or may be, forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be different from any future results, performance and achievements expressed or implied by these statements. We cannot guarantee that our forward-looking statements will turn out to be correct or that our beliefs and goals will not change. Our actual results could be very different from and worse than our expectations for various reasons. You should carefully review all information, including the discussion of risk factors under “Part I. Item 1A: Risk Factors” and “Part II. Item 7: Management’s Discussion and Analysis of Financial Condition and Results of Operations” of the Form 10-K for the year ended December 31, 2024. Any forward-looking statements in the Form 10-Q are made only as of the date hereof and, except as may be required by law, we do not have any obligation to publicly update any forward-looking statements contained in this Form 10-Q to reflect subsequent events or circumstances.
Organization
The corporate predecessor of GT Biopharma, Inc, Diagnostic Data, Inc., was incorporated in the state of California in 1965. Diagnostic Data, Inc. changed its incorporation to the state of Delaware on December 21, 1972 and changed its name to DDI Pharmaceuticals, Inc. on March 11, 1985. On September 7, 1994, DDI Pharmaceuticals, Inc. merged with International BioClinical, Inc. and Bioxytech S.A. and changed its name to OXIS International, Inc. On July 17, 2017, OXIS International, Inc. changed its name to GT Biopharma, Inc.
Overview
We are a clinical stage biopharmaceutical company focused on the development and commercialization of novel immuno-oncology products based on our proprietary Tri-specific Killer Engager (TriKE®), and Tetra-specific Killer Engager (Dual Targeting TriKE®) fusion protein immune cell engager technology platforms. Our TriKE® and Dual Targeting TriKE® platforms generate proprietary therapeutics designed to harness and enhance the cancer killing abilities of a patient’s own natural killer cells, or NK cells. Once bound to an NK cell, our moieties are designed to activate the NK cell to direct it to one or more specifically targeted proteins expressed on a specific type of cancer cell or virus infected cell, resulting in the targeted cell’s death. TriKE®s can be designed to target any number of tumor antigens, including B7-H3, HER2, CD33 and PDL1, on hematologic malignancies or solid tumors and do not require patient-specific customization. We believe our TriKE® and Dual Targeting TriKE® platforms that activate endogenous NK cells are potentially safer than T-cell immunotherapy because there is less cytokine release syndrome (CRS) and fewer neurological complications. Our preclinical data suggests that this is explained by the TriKE® dependent CD16 directed IL-15 proliferation of NK cells but little effect endogenous T cells.
We are using our TriKE® platform with the intent to bring to market immuno-oncology products that can treat a range of hematologic malignancies, solid tumors, and potentially autoimmune disorders. The platform is scalable, and we are implementing processes to produce investigational new drug (IND) ready moieties in a timely manner after a specific TriKE® conceptual design. Specific drug candidates can then be advanced into the clinic on our own or through potential collaborations with partnering companies. We believe our TriKE®s may have the ability, if approved for marketing, to be used as both monotherapy and in combination with other standard-of-care therapies.
Our initial work was conducted in collaboration with the Masonic Cancer Center at the University of Minnesota under a program led by Dr. Jeffrey Miller, Professor of Medicine, and the Interim Director at the Center. Dr. Miller, who also serves as our Consulting Senior Medical Director, is a recognized key opinion leader in the field of NK cell and IL-15 biology and their therapeutic potential. We have exclusive rights to the TriKE® platform and are generating additional intellectual property for specific moieties.
| 18 |
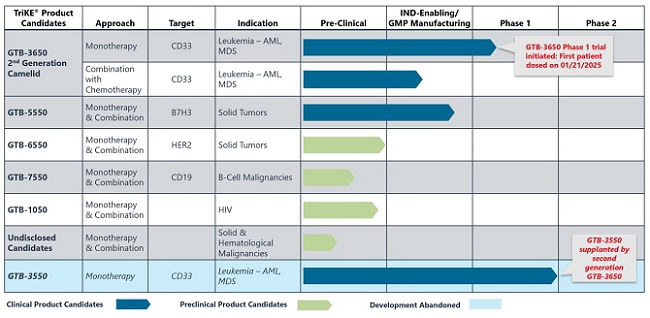
Our current product candidate pipeline is summarized in the table below:

GTB-3550
GTB-3550 was our first TriKE® product candidate and its clinical development was suspended so that we could focus resources on second-generation TriKEs®. GTB-3550 is a tri-specific killer engager (TriKE) comprised of two single-chain variable fragments (“scFv”) composed of the variable regions of the heavy and light chains of anti-CD16 and anti-CD33 antibodies and a modified form of IL-15. We studied this anti-CD16-IL-15-anti-CD33 TriKE® in CD33 positive leukemias, a marker expressed on tumor cells in acute myelogenous leukemia, or AML, and myelodysplastic syndrome, or MDS. The anti-CD33 antibody fragment in GTB-3550 was derived from the M195 humanized anti-CD33 scFv. We believe the approval of the antibody-drug conjugate gemtuzumab validates the targeting of CD33.
We previously announced the interim clinical trial results for GTB-3550, which showed significantly reduced CD 33+ bone marrow blast levels by 33.3%, 61.7%, 63.6%, 50% in Patient 5 (25 µg/kg/day), Patient 7 (50 µg/kg/day), Patient 9 (100 µg/kg/day), and Patient 11 (150 µg/kg/day), respectively. After the end of infusion, GTB-3550 and IL-15 concentrations declined rapidly with overall geometric mean terminal phase elimination half-life (T1/2) of 2.2 and 2.52 hours, respectively. There was minimal CRS resulting from hyperactivation of patient’s T-cell population at doses 5–150 µg/kg/day.
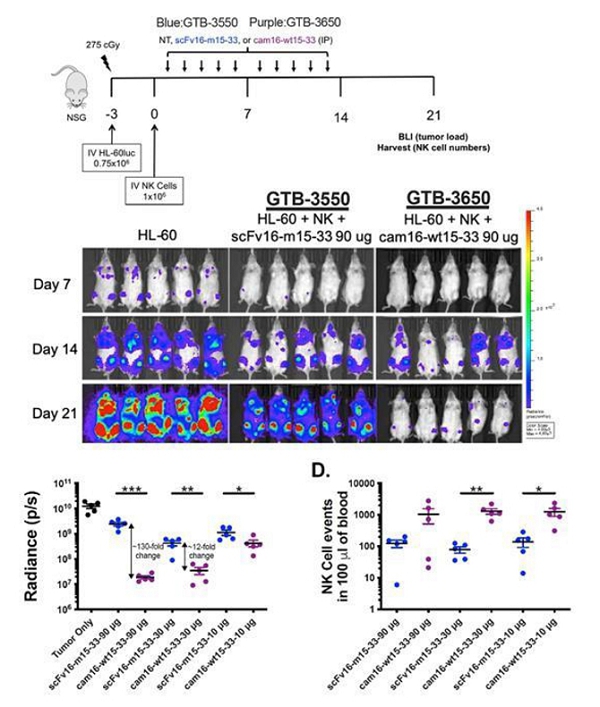
Despite the positive interim clinical trial results, GTB-3550 was replaced by a more potent next-generation camelid nanobody TriKE®, GTB-3650, that similarly targets CD33 on relapsed/refractory AML and high-risk MDS. A key difference between GTB-3550 and GTB-3650 is the incorporation of camelid antibody technology instead of a scFv; our preclinical experience showed markedly enhanced potency of TriKEs® comprised of camelid components. This is illustrated below by better tumor control of AML bearing animals with GTB-3650 (purple dots) compared to GTB-3550 (blue dots). This provided the rationale for pausing further development of GTB-3550 and moving over to solely develop the second-generation, camelid-based TriKE® platform.
| 19 |
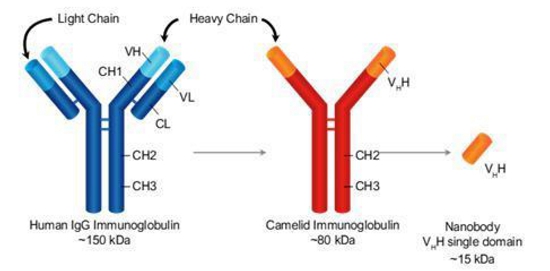
Second Generation TriKE®s Utilize Camelid Nanobody Technology
Our goal is to be a leader in immuno-oncology therapies targeting a broad range of indications including hematological malignancies and solid tumors. A key element of our strategy includes introducing a next-generation camelid nanobody platform. Camelid antibodies (often referred as nanobodies) are smaller than human immunoglobulin, consisting of two heavy chains instead of two heavy and two light chains. These nanobodies have the potential to have greater affinity to target antigens, potentially resulting in greater potency. We are utilizing this camelid antibody structure for all of our new TriKE® product candidates.
| 20 |
To develop second generation TriKE®s, we designed a new humanized CD16 engager derived from a single-domain antibody. While scFvs consist of a heavy and a light variable chain joined by a linker, single-domain antibodies consist of a single variable heavy chain capable of engaging without the need of a light chain counterpart (see figure below).
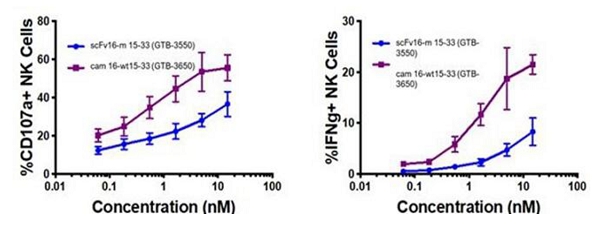
These single-domain antibodies are thought to have certain attractive features for antibody engineering, including physical stability, ability to bind deep grooves, and increased production yields, amongst others. Pre-clinical studies demonstrated increased NK cell activation against CD33+ targets including enhanced NK cell degranulation (% CD107a+) and IFNγ with the single-domain CD16 TriKE® (cam 16-wt15-33; GTB-3650) compared to the original TriKE® (scFv16-m 15-33; GTB-3550) (see figure below). This data was published by Dr. Felices M et al (2020) in Cancer Immunol Res.
CD33+ HL60 Targets in Killing Assays
The purple line represents the GTB-3650 and the blue line represents GTB-3550.
GTB-3650
GTB-3650 is a TriKE® which targets CD33 on the surface of myeloid leukemias and an agonistic camelid engager to the potent activating receptor on NK cells, CD16. Use of this engager enhances the activity of wild type IL-15 included in GTB-3650. The TriKE® approach provides a novel way to specifically target these tumors by leveraging NK cells, which have been shown to mediate relapse protection in this setting, in an anti-CD33-targeted fashion. We are advancing GTB-3650 to clinical studies based on pre-clinical data showing a marked increase in potency compared to GTB-3550, which we anticipate could lead to an enhanced efficacy signal in AML and MDS. We advanced GTB-3650 through requisite preclinical studies and filed an Investigational New Drug (IND) application with the U.S. Food and Drug Administration (FDA) in December 2023. In late June 2024, the FDA cleared our IND Application for GTB-3650. We started study enrollment targeting patients with relapsed/refractory AML and high grade MDS on January 21, 2025. This initial study is testing GTB-3650 as monotherapy testing administration 2 weeks on and two weeks off (to prevent NK cell exhaustion) for at least 2 cycles of therapy, as agreed on with the FDA.
| 21 |
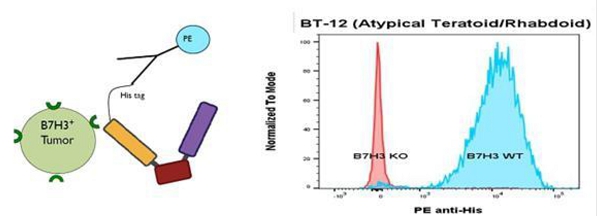
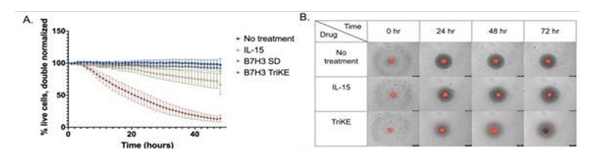
GTB-5550
GTB-5550 is a B7-H3 targeted TriKE® which targets B7-H3 on the surface of advanced solid tumors (figure above). GTB-5550 is our first dual camelid TriKE®. B7-H3 is expressed on a broad spectrum of solid tumor malignancies, allowing our team to target these malignancies through GTB-5550. Pre-clinical work has shown that this molecule has NK-cell targeted activity against a variety of solid tumors, including head and neck cancer squamous cell carcinoma (figure below), prostate cancer, breast cancer, ovarian cancer, glioblastoma, and lung cancer (amongst others). We are advancing GTB-5550 through preclinical studies and initiated a GMP manufacturing campaign in anticipation of filing an IND. A pre-IND packet was submitted to the FDA in October 2023 with a written response from the FDA in December 2023. The main question from the FDA was regarding pre-clinical toxicology and a pivot to subcutaneous dosing. The initial trial is designed as a basket trial for patients with B7-H3+ solid tumors using Monday through Friday dosing (2 weeks on and 2 weeks off to prevent immune exhaustion), and is dependent on manufacturing of clinical materials.
GTB-7550
GTB-7550 TriKE® is a product candidate in development for the treatment of lupus and other autoimmune disorders. GTB-7550 TriKE® is a tri-specific molecule composed of a camelid nanobody that binds the CD16 receptor on NK cells, a scFv engager against CD19 on malignant and normal B cells, and a human IL-15 sequence between them.
Published data shows that GTB-7550 effectively targets CD19+ malignant cell lines and primary chronic lymphocytic leukemia (CLL). Preliminary data shows that GTB-7550 can target and eliminate normal B cells, which we are continuing to test in mice. We are currently exploring and assessing potential manufacturers of GTB-7550.
Critical Accounting Policies and Estimates
The preparation of our financial statements in conformity with accounting principles generally accepted in the United States, or GAAP, requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. When making these estimates and assumptions, we consider our historical experience, our knowledge of economic and market factors and various other factors that we believe to be reasonable under the circumstances. Actual results may differ under different estimates and assumptions. The accounting estimates and assumptions discussed in this section are those that we consider to be the most critical to gain an understanding of our financial statements because they inherently involve significant judgments and uncertainties.
| 22 |
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include accruals for potential liabilities, assumptions used in deriving the fair value of warrant liabilities, valuation of equity instruments issued for services, and valuation of deferred tax assets. Actual results could differ from those estimates.
Warrant Liability
We evaluate our financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations.
Our use of derivative financial instruments is generally limited to warrants issued by us that do not meet the criteria for equity treatment and are recorded as liabilities. We do not use financial instruments or derivatives for any trading purposes.
Stock-Based Compensation
We periodically issue stock-based compensation to officers, directors, employees, and consultants for services rendered. Such issuances vest and expire according to terms established at the issuance date.
Stock-based payments made to officers, directors, employees, and consultants in exchange for goods and services, including grants of employee stock options, are recognized in the financial statements based on their grant date fair values in accordance with ASC 718, Compensation-Stock Compensation. Stock based payments to officers, directors, employees, and consultants, which are generally time vested, are measured at the grant date fair value and depending on the conditions associated with the vesting of the award, compensation cost is recognized on a straight-line or graded basis over the vesting period. Recognition of compensation expense for non-employees is in the same period and manner as if we had paid cash for the services. The fair value of stock options granted is estimated using the Black-Scholes option-pricing model, which uses certain assumptions related to risk-free interest rates, expected volatility, expected life, and future dividends. The assumptions used in the Black-Scholes option pricing model could materially affect compensation expense recorded in future periods.
Results of Operations
Comparison of the Three Months Ended March 31, 2025 and 2024
Operating Expenses
| Three Months Ended March 31, | ||||||||||||||||
| 2025 | 2024 | $ Change | % Change | |||||||||||||
| Operating Expenses: | ||||||||||||||||
| Research and development | $ | 1,099,000 | $ | 777,000 | $ | 322,000 | 41 | % | ||||||||
| Selling, general and administrative | 830,000 | 2,212,000 | (1,382,000 | ) | (62 | )% | ||||||||||
| Stock compensation | 3,000 | 102,000 | (99,000 | ) | (97 | )% | ||||||||||
| Total Operating Expenses | $ | 1,932,000 | $ | 3,091,000 | $ | (1,159,000 | ) | (37 | )% | |||||||
Research and Development Expenses
Research and development expenses increased by approximately $322,000 for the three months ended March 31, 2025 compared to the same prior year period, primarily due to an increase in scientific research costs.
Research and development expenses relate to our continued licensing, development and production of our most advanced TriKE® product candidates GTB-3650 and GTB-5550 along with the progression on other promising candidates. In late June 2024, we received clearance from the FDA with respect to our IND Application in relation to our next generation GTB-3650 camelid nanobody product. Study enrollment began in early 2025 and we are advancing into the clinic, enrolling patients, and performing tests for data collection throughout the year. We have paused the final phase of product development of GTB-5550 to reduce costs.
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses decreased by approximately $1.4 million for the three months ended March 31, 2025, compared to the same prior year period. The decrease was primarily due to cost reduction measures including a reduction in legal fees, consulting fees, and salaries.
| 23 |
Other Income (Expense)
| Three Months Ended March 31, | ||||||||||||||||
| 2025 | 2024 | $ Change | % Change | |||||||||||||
| Other Income (Expense): | ||||||||||||||||
| Interest income | $ | 32,000 | $ | 142,000 | $ | (110,000 | ) | (77 | )% | |||||||
| Change in fair value of warrant liability | 126,000 | 658,000 | (532,000 | ) | (81 | )% | ||||||||||
| Unrealized gain (loss) on marketable securities | — | (2,000 | ) | 2,000 | 100 | % | ||||||||||
| Gain on settlement of vendor payable | 998,000 | — | 998,000 | — | % | |||||||||||
| Other | — | 27,000 | (27,000 | ) | (100 | )% | ||||||||||
| Total Other Income (Expense) | $ | 1,156,000 | $ | 825,000 | $ | 331,000 | 40 | % | ||||||||
Interest Income
Interest income decreased by $110,000 for the three months ended March 31, 2025 compared to the same prior year period, due to lower money market fund and short term investment balances.
Change in Fair Value of Warrant Liability
The change in fair value of warrant liability decreased by approximately $532,000, for the three months ended March 31, 2025 compared to the same prior year period, resulting from a reduction in our warrant liability primarily due to the decline in the Company’s stock price at March 31, 2025 as compared to the prior comparable periods.
Gain on Settlement of Vendor Payable
In March 2025, a legal services firm currently engaged by the Company agreed to reduce the Company’s prior year unpaid fees by approximately $1 million. The Company classified this transaction as other income during the period ended March 31, 2025.
Net Loss
| Three Months Ended March 31, | ||||||||||||||||
| 2025 | 2024 | $ Change | % Change | |||||||||||||
| Net Loss | $ | (776,000 | ) | $ | (2,266,000 | ) | $ | 1,490,000 | 66 | % | ||||||
Net loss decreased by $1,490,000 for the three months ended March 31, 2025 compared to the same prior year period, primarily due to a decrease in legal fees, consulting fees, and salaries, and the reduction of prior year unpaid legal fees, as described above.
Liquidity and Going Concern Analysis
The accompanying unaudited condensed financial statements have been prepared assuming that we will continue as a going concern. We do not have any product candidates approved for sale and have not generated any revenue from our product sales. We have sustained operating losses since inception, and we expect such losses to continue into the foreseeable future. Historically, we have financed our operations through public and private sales of common stock, issuance of preferred and common stock, issuance of convertible debt instruments, and strategic collaborations. For the three months ended March 31, 2025, we recorded a net loss of approximately $800,000 and used cash in operations of approximately $2.2 million, and had a stockholders’ deficit of approximately $1 million as of that date. These factors raise substantial doubt about our ability to continue as a going concern within one year of the date that the financial statements are issued. In addition, the Company’s independent registered public accounting firm, in its report on the Company’s December 31, 2024, financial statements, raised substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
The unaudited condensed financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern. Accordingly, the unaudited condensed financial statements have been prepared on a basis that assumes we will continue as a going concern, and which contemplates the realization of assets and satisfaction of liabilities and commitments in the ordinary course of business.
We have evaluated the significance of the uncertainty regarding our financial condition in relation to our ability to meet our obligations, which has raised substantial doubt about our ability to continue as a going concern. While it is very difficult to estimate our future liquidity requirements we believe if we are unable to obtain additional financing, existing cash resources will not be sufficient to enable us to fund the anticipated level of operations through one year from the date the accompanying unaudited condensed financial statements are issued. There can be no assurances that we will be able to secure additional financing on acceptable terms. In the event that we do not secure additional financing, we will be forced to delay, reduce, or eliminate some or all of our discretionary spending, which could adversely affect our business prospects, ability to meet long-term liquidity needs and the ability to continue operations.
| 24 |
Cash Flows
| Three Months Ended March 31, | ||||||||
| 2025 | 2024 | |||||||
| Statements of Cash Flow Data: | ||||||||
| Net cash used in operating activities | $ | (2,202,000 | ) | $ | (4,163,000 | ) | ||
| Net cash provided by investing activities | — | 5,034,000 | ||||||
| Net cash provided by financing activities | 616,000 | — | ||||||
| Net increase (decrease) in cash and cash equivalents and restricted cash | (1,586,000 | ) | 871,000 | |||||
| Cash and cash equivalents and restricted cash, beginning of period | 4,044,000 | 1,079,000 | ||||||
| Cash and cash equivalents and restricted cash, end of period | $ | 2,458,000 | $ | 1,950,000 | ||||
Operating Activities
Net cash used in operating activities was approximately $2.2 million for the three months ended March 31, 2025, and was primarily due to a decrease in accounts payable and accrued expenses of approximately $1.3 million, and a net loss of $776,000.
Net cash used in operating activities was $4.2 million for the three months ended March 31, 2024, and was primarily due to a net loss of $2.3 million, a decrease in accounts payable and accrued expenses of $1.3 million, and a decrease in the fair value of warrant liability of $658,000.
Investing Activities
Net cash provided by financing activities was approximately $5.0 million for the three months ended March 31, 2024, resulted from proceeds of the sale of short-term investments.
Financing Activities
Net cash provided by financing activities was $616,000 for the three months ended March 31, 2025, resulted from the exercise of warrants for cash and inducement warrants.
Working Capital (Deficit)
The following table summarizes total current assets, liabilities, and working capital (deficit) for the periods ended March 31, 2025 and December 31, 2024:
| As of | ||||||||||||
March 31, 2025 | December
31, 2024 | Increase/(Decrease) | ||||||||||
| Current assets | $ | 2,658,000 | $ | 4,232,000 | $ | (1,574,000 | ) | |||||
| Current liabilities | $ | 3,638,000 | $ | 5,902,000 | $ | (2,264,000 | ) | |||||
| Working capital (deficit) | $ | (980,000 | ) | $ | (1,670,000 | ) | $ | 690,000 | ||||
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements as of March 31, 2025.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Our Company qualifies as a smaller reporting company, as defined in 17 C.F.R. §229.10(f)(1) and is not required to provide information for this Item.
| 25 |
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer evaluated the effectiveness of our “disclosure controls and procedures” (as such term is defined in Rules 13a-15(e) and 15d-15(e) of the United States Securities Exchange Act of 1934, as amended), as of March 31, 2025. Based on that evaluation, we have concluded that our disclosure controls and procedures were effective as of March 31, 2025.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of, a company’s principal executive and principal accounting officers and effected by a company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with GAAP and includes those policies and procedures that:
| ● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets | |
| ● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with GAAP, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and | |
| ● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Inherent Limitations on the Effectiveness of Controls
Management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control systems are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in a cost-effective control system, no evaluation of internal control over financial reporting can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been or will be detected.
These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of a simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Changes in Internal Control Over Financial Reporting
No changes in our internal controls over financial reporting were made during our most recent fiscal quarter that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
| 26 |
PART II. OTHER INFORMATION
Item 1. Legal Proceedings
Ohri Matter
On July 22, 2024, the Company filed an AAA Arbitration Demand against Manu Ohri, its former Chief Financial Officer. In the Demand, the Company asserts claims against Mr. Ohri for breach of his fiduciary duties and breach of contract and seeks a declaratory judgment providing that the Company may characterize Mr. Ohri’s termination as “for cause” under his Employment Agreement, and that the Company may revoke the Separation Agreement entered into between the Company and Mr. Ohri prior to the Company learning of Mr. Ohri’s breaches. In addition to the declaratory judgment, the Company seeks damages arising from Mr. Ohri’s violations, and attorneys’ fees and any forum and arbitration fees. On September 3, 2024, Mr. Ohri filed both a general denial of the Company’s claims against him and counterclaims for breach of his Employment Agreement and Separation Agreement. A final hearing is scheduled for June 10th through June 12th, 2025 in Los Angeles, California. At this stage in the proceedings the Company is not able to determine the probability of the outcome of this matter or a range of reasonably expected losses, if any. The Company believes it has recorded an appropriate accrual for the matter.
TWF Global Matter
On May 24, 2023, TWF Global, LLC (“TWF”) filed a Complaint in the California Superior Court for the County of Los Angeles naming the Company as defendant. The Complaint alleges that TWF is the holder of two Convertible Promissory Notes (“Notes”) and that the Company did not deliver shares of common stock due on conversion in February 2021. TWF was seeking per diem liquidated damages based on the terms of alleged Notes. On July 14, 2023, the Company filed a motion to dismiss for improper forum because the terms of the Notes, as alleged, require disputes to be filed in New York state and federal courts. TWF voluntarily dismissed its Complaint before the California Superior Court of Los Angeles without prejudice. The Company subsequently filed a Summons and Complaint for Interpleader against TWF and Z-One LLC before the Supreme Court of the State of New York County of New York, asking the Supreme Court to determine if the Company’s shares of common stock are properly registered to TWF or Z-One LLC, as both of these entities have made conflicting demands for registration of the shares of common stock. On February 5, 2024, the Company filed a motion for entry of default against TWF, seeking an order directing the Company to register the shares of common stock in the name of Z-One and that the Company be released from all associated liability and claims. The Court denied the motion without prejudice and agreed to reconsider the motion without further briefing upon the filing of a supplemental party affidavit. On May 9, 2024, Z-One filed a motion for summary judgement seeking dismissal of the action, representing that Z-One and TWF have settled their dispute over the entitlement to the Company’s shares of common stock and there is no remaining dispute before the Court. On May 21, 2024, the Company filed a supplemental affidavit in support of its motion for entry of default. On November 14, 2024, the Court held a hearing on the parties’ motions, at which the Court found that the motion for entry of default was mooted by the settlement agreement between Z-One and TWF. The Court ordered that the case be dismissed. On February 17, 2025, Z-One, LLC filed a Summons with Notice in the Supreme Court of the State of New York, County of New York. Z-One alleges that it was assigned TWF’s rights under the Notes and seeks enforcement and damages. The Company intends to demand a complaint, and seek dismissal of the case. The Company believes that the claims related to the Notes are without merit, and will continue to vigorously defend against these claims.
Item 5. Other Information
Rule 10b5-1 Trading Plans
During the three months ended March 31, 2025, none of our directors or officers (as defined in Rule 16a-1(f) under the Securities Exchange Act of 1934, as amended) adopted, amended, or terminated a “Rule 10b5-1 trading arrangement” or a “non-Rule 10b5-1 trading arrangement,” as each term is defined in Item 408 of Regulation S-K.
| 27 |
Item 6. Exhibits
| * | This certification shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that Section, nor shall it be deemed to be incorporated by reference into any filing under the Securities Act or the Exchange Act. |
| 28 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| GT BIOPHARMA, INC. | ||
| Dated: May 15, 2025 | By: | /s/ Michael Breen |
| Michael Breen | ||
Chief Executive Officer and Executive Chairman of the Board | ||
| (Principal Executive Officer) | ||
| Dated: May 15, 2025 | By: | /s/ Alan Urban |
| Alan Urban | ||
| Chief Financial Officer & Secretary | ||
| (Principal Financial and Accounting Officer) |
| 29 |