UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
| (Mark One) | |
| ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For
the fiscal year ended: or | |
| TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
For the transition period from _____________ to _____________
Commission
File Number:
(Exact name of Registrant as specified in its charter)
| (State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
N/A1
(Address of principal executive offices)
(Registrant’s telephone number including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Securities | Trading Symbol(s) | Exchanges on which Registered | ||
Indicate
by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate
by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate
by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange
Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2)
has been subject to such filing requirements for the past 90 days.
Indicate
by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule
405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant
was required to submit and post such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ |
| ☒ | Smaller reporting company | ||
| Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate
by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness
of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered
public accounting firm that prepared or issued its audit report.
If
securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant
included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
Indicate
by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No
The
aggregate market value of the registrant’s common stock, $0.001 par value per share, held by non-affiliates on June 30, 2024 was
approximately $
| 1 | Effective as of
July 1, 2024, the Company became a fully remote company. We do not maintain a principal executive office. For purposes of compliance
with applicable requirements of the Securities Act of 1933, as amended, and the Securities Exchange Act of 1934, as amended, any stockholder
communication required to be sent to the Company’s principal executive offices may be directed to |
GT Biopharma, Inc.
FORM 10-K
TABLE OF CONTENTS
SUMMARY RISK FACTORS
Our business involves significant risks. Below is a summary of the material risks that our business faces, which makes an investment in our securities speculative and risky. This summary does not address all these risks. These risks are more fully described below under the heading “Risk Factors” in Part I, Item 1A of this annual report on Form 10-K. Before making investment decisions regarding our securities, you should carefully consider these risks. The occurrence of any of the events or developments described below could have a material adverse effect on our business, results of operations, financial condition, prospects and stock price. In such event, the market price of our securities could decline, and you could lose all or part of your investment. In addition, there are also additional risks not described below that are either not presently known to us or that we currently deem immaterial, and these additional risks could also materially impair our business, operations, or market price of our common stock.
● Our financial condition raises substantial doubt as to our ability to continue as a going concern.
● Our business is at an early stage of development and we may not develop therapeutic products that can be commercialized.
● We have a history of operating losses and we expect to continue to incur losses for the foreseeable future. We may never generate revenue or achieve profitability.
● We will need additional capital to conduct our operations and develop our products, and our ability to obtain the necessary funding is uncertain.
● Our current and future indebtedness may impose significant operating and financial restrictions on us and affect our ability to access liquidity.
● The cost of our research and development programs may be significantly higher than expected, and there is no assurance that they will successful in a timely manner, or at all.
● If our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not be able to compete effectively in our market and our business would be harmed.
● Claims that we infringe the intellectual property rights of others may prevent or delay our drug discovery and development efforts.
● We may desire, or be forced, to seek additional licenses to use intellectual property owned by third parties, and such licenses may not be available on commercially reasonable terms, or at all.
● If we are unsuccessful in obtaining or maintaining patent protection for intellectual property in development or licensed from third parties, our business and competitive position would be harmed.
● If we fail to meet our obligations under our license agreements, we may lose our rights to key technologies on which our business depends.
● Our reliance on the activities of our non-employee consultants, research institutions and scientific contractors, whose activities are not wholly within our control, may lead to delays in development of our proposed products.
● Clinical drug development is costly, time-consuming and uncertain, and we may suffer setbacks in our clinical development program that could harm our business.
● If we experience delays or difficulties in the enrollment of patients in clinical trials, those clinical trials could take longer than expected to complete and our receipt of necessary regulatory approvals could be delayed or prevented.
● Obtaining regulatory approval, even after clinical trials that are believed to be successful, is an uncertain process.
● We will continue to be subject to extensive FDA regulation following any product approvals, and if we fail to comply with these regulations, we may suffer a significant setback in our business.
● Many of our business practices are subject to scrutiny and potential investigation by regulatory and government enforcement authorities, as well as to lawsuits brought by private citizens under federal and state laws. We could become subject to investigations, and our failure to comply with applicable law or an adverse decision in lawsuits may result in adverse consequences to us. If we fail to comply with U.S. healthcare laws, we could face substantial penalties and financial exposure, and our business, operations and financial condition could be adversely affected.
● Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
● We may expend our limited resources to pursue a particular product candidate or indication that does not produce any commercially viable products and may fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
● Our products may be expensive to manufacture, and they may not be profitable if we are unable to control the costs to manufacture them.
● We currently lack manufacturing capabilities to produce our therapeutic product candidates at commercial-scale quantities and do not have an alternate manufacturing supply, which would negatively impact our ability to meet any demand for the product.
● Our business is based on novel technologies that are inherently expensive and risky and may not be understood by or accepted in the marketplace, which could adversely affect our future value.
● We could be subject to product liability lawsuits based on the use of our product candidates in clinical testing or, if obtained, following marketing approval and commercialization. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to cease clinical testing or limit commercialization of our product candidates.
● We rely on third parties to supply candidates for clinical testing and to conduct preclinical and clinical trials of our product candidates. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates. As a result, our business could be substantially harmed.
● Our failure to maintain compliance with the Nasdaq Capital Market’s (“Nasdaq”) continued listing requirements could result in the delisting of our common stock.
● There has been a limited public market for our common stock, and we do not know whether one will develop to provide adequate liquidity. Furthermore, the trading price for our common stock, should an active trading market develop, may be volatile and could be subject to wide fluctuations in per-share price.
● Because our common stock may be deemed a “penny” stock, an investment in our common stock should be considered high-risk and subject to marketability restrictions.
PART I
CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K, including any documents which may be incorporated by reference into this Annual Report, contains “Forward-Looking Statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical fact are “Forward-Looking Statements” for purposes of these provisions, including our plans of operation, any projections of revenues or other financial items, any statements of the plans and objectives of management for future operations, any statements concerning proposed new products or services, any statements regarding future economic conditions or performance, and any statements of assumptions underlying any of the foregoing. All Forward-Looking Statements included in this document are made as of the date hereof and are based on information available to us as of such date. We assume no obligation to update any Forward-Looking Statement. In some cases, Forward-Looking Statements can be identified by the use of terminology such as “may,” “will,” “expects,” “plans,” “anticipates,” “intends,” “believes,” “estimates,” “potential,” or “continue,” or the negative thereof or other comparable terminology. Although we believe that the expectations reflected in the Forward-Looking Statements contained herein are reasonable, there can be no assurance that such expectations or any of the Forward-Looking Statements will prove to be correct, and actual results could differ materially from those projected or assumed in the Forward-Looking Statements. Future financial condition and results of operations, as well as any Forward-Looking Statements are subject to inherent risks and uncertainties, including any other factors referred to in our press releases and reports filed with the Securities and Exchange Commission. All subsequent Forward-Looking Statements attributable to the company or persons acting on its behalf are expressly qualified in their entirety by these cautionary statements. Additional factors that may have a direct bearing on our operating results are described under “Risk Factors” and elsewhere in this Annual Report on Form 10-K.
Introductory Comment
Throughout this Annual Report on Form 10-K, the terms “GT Biopharma,” “GTBP,” “we,” “us,” “our,” “the company” and “our company” refer to GT Biopharma, Inc., a Delaware corporation formerly known as DDI Pharmaceuticals, Inc., Diagnostic Data, Inc. and OXIS International, Inc., together with our former wholly-owned subsidiaries, Oxis Biotech, Inc. and Georgetown Translational Pharmaceuticals, Inc., which were both dissolved on October 22, 2024.
ITEM 1. BUSINESS
We are a clinical stage biopharmaceutical company focused on the development and commercialization of novel immuno-oncology products based on our proprietary Tri-specific Killer Engager (TriKE®), and Tetra-specific Killer Engager (Dual Targeting TriKE®) fusion protein immune cell engager technology platforms. Our TriKE® and Dual Targeting TriKE® platforms generate proprietary therapeutics designed to harness and enhance the cancer killing abilities of a patient’s own natural killer cells, or NK cells. Once bound to an NK cell, our moieties are designed to activate the NK cell to direct it to one or more specifically targeted proteins expressed on a specific type of cancer cell or virus infected cell, resulting in the targeted cell’s death. TriKE®s can be designed to target any number of tumor antigens, including B7-H3, HER2, CD33 and PDL1, on hematologic malignancies or solid tumors and do not require patient-specific customization. We believe our TriKE® and Dual Targeting TriKE® platforms that activate endogenous NK cells are potentially safer than T-cell immunotherapy because there is less cytokine release syndrome (CRS) and fewer neurological complications. Our preclinical data suggests that this is explained by the TriKE® dependent CD16 directed IL-15 proliferation of NK cells but little effect endogenous T cells.
We are using our TriKE® platform with the intent to bring to market immuno-oncology products that can treat a range of hematologic malignancies, solid tumors, and potentially autoimmune disorders. The platform is scalable, and we are implementing processes to produce investigational new drug (IND) ready moieties in a timely manner after a specific TriKE® conceptual design. Specific drug candidates can then be advanced into the clinic on our own or through potential collaborations with partnering companies. We believe our TriKE®s may have the ability, if approved for marketing, to be used as both monotherapy and in combination with other standard-of-care therapies.
Our initial work was conducted in collaboration with the Masonic Cancer Center at the University of Minnesota under a program led by Dr. Jeffrey Miller, Professor of Medicine, and the Interim Director at the Center. Dr. Miller, who also serves as our Consulting Senior Medical Director, is a recognized key opinion leader in the field of NK cell and IL-15 biology and their therapeutic potential. We have exclusive rights to the TriKE® platform and are generating additional intellectual property for specific moieties.
| 1 |
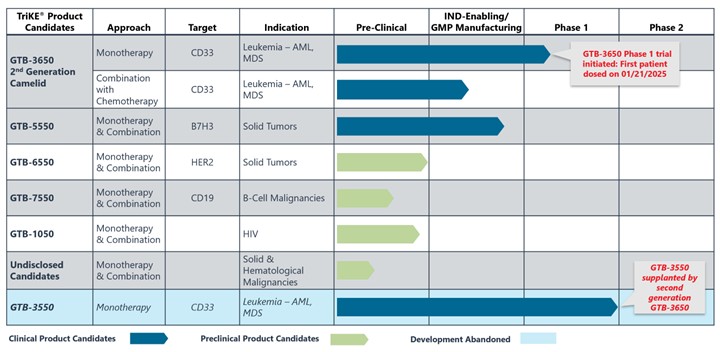
Product Pipeline
Our current product candidate pipeline is summarized in the table below:

GTB-3550
GTB-3550 was our first TriKE® product candidate and its clinical development was suspended so that we could focus resources on second-generation TriKEs®. GTB-3550 is a tri-specific killer engager (TriKE) comprised of two single-chain variable fragments (“scFv”) composed of the variable regions of the heavy and light chains of anti-CD16 and anti-CD33 antibodies and a modified form of IL-15. We studied this anti-CD16-IL-15-anti-CD33 TriKE® in CD33 positive leukemias, a marker expressed on tumor cells in acute myelogenous leukemia, or AML, and myelodysplastic syndrome, or MDS. The anti-CD33 antibody fragment in GTB-3550 was derived from the M195 humanized anti-CD33 scFv We believe the approval of the antibody-drug conjugate gemtuzumab validates the targeting of CD33.
We previously announced the interim clinical trial results for GTB-3550, which showed significantly reduced CD 33+ bone marrow blast levels by 33.3%, 61.7%, 63.6%, 50% in Patient 5 (25 µg/kg/day), Patient 7 (50 µg/kg/day), Patient 9 (100 µg/kg/day), and Patient 11 (150 µg/kg/day), respectively. After the end of infusion, GTB-3550 and IL-15 concentrations declined rapidly with overall geometric mean terminal phase elimination half-life (T1/2) of 2.2 and 2.52 hours, respectively. There was minimal CRS resulting from hyperactivation of patient’s T-cell population at doses 5–150 µg/kg/day.
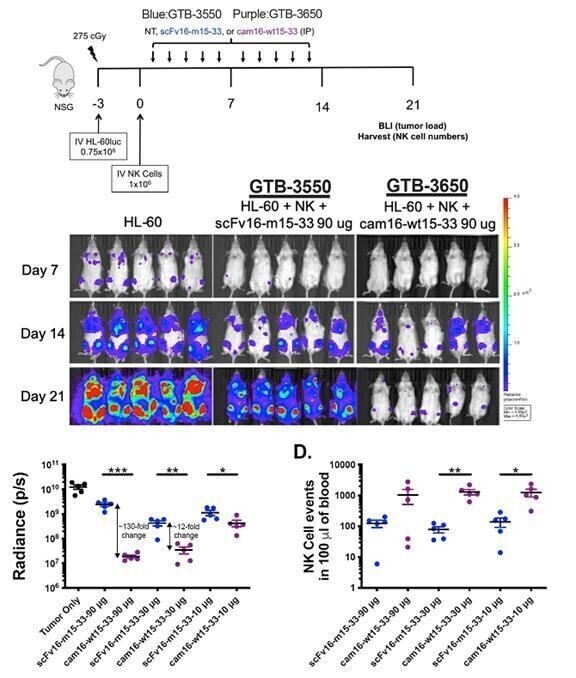
Despite the positive interim clinical trial results, GTB-3550 was replaced by a more potent next-generation camelid nanobody TriKE®, GTB-3650, that similarly targets CD33 on relapsed/refractory AML and high-risk MDS. A key difference between GTB-3550 and GTB-3650 is the incorporation of camelid antibody technology instead of a scFv; our preclinical experience showed markedly enhanced potency of TriKEs® comprised of camelid components. This is illustrated below by better tumor control of AML bearing animals with GTB-3650 (purple dots) compared to GTB-3550 (blue dots). This provided the rationale for pausing further development of GTB-3550 and moving over to solely develop the second-generation, camelid-based TriKE® platform.
| 2 |
Second Generation TriKE®s Utilize Camelid Nanobody Technology
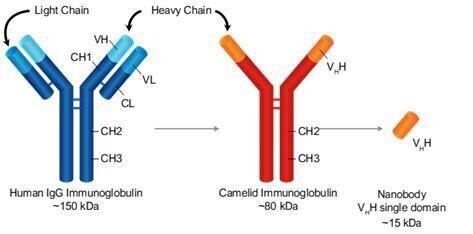
Our goal is to be a leader in immuno-oncology therapies targeting a broad range of indications including hematological malignancies and solid tumors. A key element of our strategy includes introducing a next-generation camelid nanobody platform. Camelid antibodies (often referred as nanobodies) are smaller than human immunoglobulin, consisting of two heavy chains instead of two heavy and two light chains. These nanobodies have the potential to have greater affinity to target antigens, potentially resulting in greater potency. We are utilizing this camelid antibody structure for all of our new TriKE® product candidates.
To develop second generation TriKE®s, we designed a new humanized CD16 engager derived from a single-domain antibody. While scFvs consist of a heavy and a light variable chain joined by a linker, single-domain antibodies consist of a single variable heavy chain capable of engaging without the need of a light chain counterpart (see figure below).
| 3 |
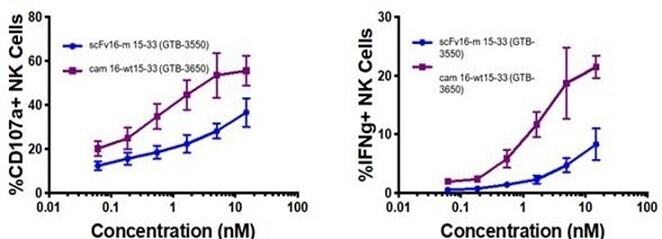
These single-domain antibodies are thought to have certain attractive features for antibody engineering, including physical stability, ability to bind deep grooves, and increased production yields, amongst others. Pre-clinical studies demonstrated increased NK cell activation against CD33+ targets including enhanced NK cell degranulation (% CD107a+) and IFNγ with the single-domain CD16 TriKE® (cam 16-wt15-33; GTB-3650) compared to the original TriKE® (scFv16-m 15-33; GTB-3550) (see figure below). This data was published by Dr. Felices M et al (2020) in Cancer Immunol Res.
CD33+ HL60 Targets in Killing Assays
The purple line represents the GTB-3650 and the blue line represents GTB-3550.
GTB-3650
GTB-3650 is a TriKE® which targets CD33 on the surface of myeloid leukemias and an agonistic camelid engager to the potent activating receptor on NK cells, CD16. Use of this engager enhances the activity of wild type IL-15 included in GTB-3650. The TriKE® approach provides a novel way to specifically target these tumors by leveraging NK cells, which have been shown to mediate relapse protection in this setting, in an anti-CD33-targeted fashion. We are advancing GTB-3650 to clinical studies based on pre-clinical data showing a marked increase in potency compared to GTB-3550, which we anticipate could lead to an enhanced efficacy signal in AML and MDS. We advanced GTB-3650 through requisite preclinical studies and filed an Investigational New Drug (IND) application with the U.S. Food and Drug Administration (FDA) in December 2023. In late June 2024, the FDA cleared our IND Application for GTB-3650. We started study enrollment targeting patients with relapsed/refractory AML and high grade MDS on January 21, 2025. This initial study is testing GTB-3650 as monotherapy testing administration 2 weeks on and two weeks off (to prevent NK cell exhaustion) for at least 2 cycles of therapy, as agreed on with the FDA.
| 4 |
GTB-5550
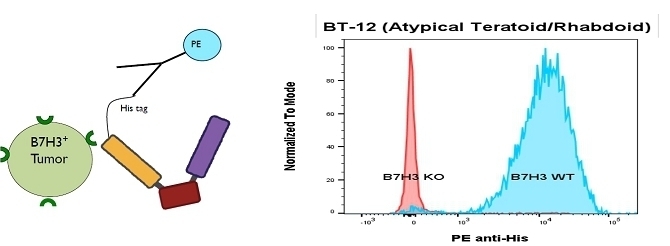
GTB-5550 is a B7-H3 targeted TriKE® which targets B7-H3 on the surface of advanced solid tumors (figure above). GTB-5550 is our first dual camelid TriKE®. B7-H3 is expressed on a broad spectrum of solid tumor malignancies, allowing our team to target these malignancies through GTB-5550. Pre-clinical work has shown that this molecule has NK-cell targeted activity against a variety of solid tumors, including head and neck cancer squamous cell carcinoma (figure below), prostate cancer, breast cancer, ovarian cancer, glioblastoma, and lung cancer (amongst others). We are advancing GTB-5550 through preclinical studies and initiated a GMP manufacturing campaign in anticipation of filing an IND. A pre-IND packet was submitted to the FDA in October 2023 with a written response from the FDA in December 2023. The main question from the FDA was regarding pre-clinical toxicology and a pivot to subcutaneous dosing. The initial trial is designed as a basket trial for patients with B7-H3+ solid tumors using Monday through Friday dosing (2 weeks on and 2 weeks off to prevent immune exhaustion), and is dependent on manufacturing of clinical materials.
GTB-7550
GTB-7550 TriKE® is a product candidate in development for the treatment of lupus and other autoimmune disorders. GTB-7550 TriKE® is a tri-specific molecule composed of a camelid nanobody that binds the CD16 receptor on NK cells, a scFv engager against CD19 on malignant and normal B cells, and a human IL-15 sequence between them.
Published data shows that GTB-7550 effectively targets CD19+ malignant cell lines and primary chronic lymphocytic leukemia (CLL). Preliminary data shows that GTB-7550 can target and eliminate normal B cells, which we are continuing to test in mice. We are currently exploring and assessing potential manufacturers of GTB-7550.
Oncology Markets
Acute Myeloid Leukemia and Myelodysplastic Syndromes
AML is a heterogeneous hematologic stem cell malignancy in adults with an incidence rate of 4.3% per 100,000 populations. The median age at the time of diagnosis is 68 years. AML is an aggressive disease and is fatal without anti-leukemic treatment. AML is the most common form of adult leukemia in the U.S. These patients will require frontline therapy, usually chemotherapy including cytarabine and an anthracycline, a therapy that has not changed in over 40 years. Myelodysplastic syndromes are a heterogeneous group of myeloid neoplasms characterized by dysplastic features of erythroid/myeloid/megakaryocytic lineages, progressive bone marrow failure, a varying percentage of blast cells, and enhanced risk to evolve into acute myeloid leukemia. It is estimated that over 10,000 new cases of MDS are diagnosed each year and there are minimal treatment options; other estimates have put this number higher. In addition, the incidence of MDS is rising for unknown reasons.
| 5 |
B7-H3 Positive Solid Tumors
The B7-H3 protein, which functions as a checkpoint inhibitor, has been identified in many of the most common solid tumor cancers, including but not limited to bladder, breast, cervical, colorectal, endometrial, esophageal, gastric, glioma, kidney, liver, lung, pancreatic, prostate, head and neck cancer, and melanoma. In recent studies, B7-H3 has been identified as a critical promoter of tumor cell proliferation, migration, invasion, epithelial-to-mesenchymal transition, cancer stemness and drug resistance. Because this protein does not seem to be expressed in normal cells, this makes it an attractive target for therapeutic intervention.
Manufacturing
We do not currently own or operate manufacturing facilities for the production of clinical or commercial quantities of any of our product candidates. We rely on third-party contract manufacturing operations, including Cytovance Biologics, Inc. (“Cytovance”), a related party, to produce and/or test our compounds and expect to continue to do so to meet the preclinical and clinical requirements of our potential product candidates as well as for our future commercial needs. We require in our manufacturing and processing agreements that third-party product manufacturers produce intermediates, active pharmaceutical ingredients, or API, and finished products in accordance with the FDA’s current Good Manufacturing Practices (cGMP), and all other applicable laws and regulations. We maintain confidentiality agreements with potential and existing manufacturers to protect our proprietary rights related to our drug candidates.
Cytovance Biologics, Inc., a Related Party
In October 2020, the Company entered into a Master Services Agreement with Cytovance Biologics, Inc. (“Cytovance”), to perform biologic development and manufacturing services, and to produce and test compounds used in the Company’s potential product candidates. The Company subsequently executed numerous Statements of Work (“SOWs”) for the research and development of products for use in clinical trials.
On August 24, 2022, the Company entered into a Settlement and Investment Agreement with Cytovance that amended existing SOWs and allowed for future invoices to be settled in in a combination of cash and issuance of the Company’s common stock. The Agreement also set Cytovance’s beneficial ownership limitation at 4.9% of the issued and outstanding shares of the Company’s common stock.
On April 25, 2024, the Company entered into an Amendment to the Settlement and Investment Agreement with Cytovance that increased Cytovance’s beneficial ownership limitation to 9.9% of the issued and outstanding shares of the Company’s common stock.
During the years ended December 31, 2024 and 2023, the Company recognized research and development expenses of $2,335,000 and $4,584,000, respectively and made cash payments amounting to $3,857,000 and $2,213,000, respectively to Cytovance. In addition, the Company issued 127,597 and 57,437 shares of common stock to Cytovance to settle accounts payable valued at approximately $810,000 and $1,120,000, respectively.
On June 30, 2024, Cytovance became a related party as their beneficial ownership exceeded 5% of the issued and outstanding shares of the Company’s common stock.
As of December 31, 2024 the Company’s commitments in relation to unbilled and unaccrued SOWs and any related Change Orders from Cytovance for services that have not yet been rendered as of December 31, 2024, amounted to approximately $1.1 million.
TriKE® Patents and Trademarks
On August 24, 2021, two patents were issued by the US Patent Office covering our pipeline of clinical and non-clinical product candidates consisting of tri-specific killer engagers, or TriKE®s, designed to target natural killer, or NK, cells and tumor or virus infected cells forming an immune synapse between the NK cell and the tumor cell thereby inducing NK cell activation at that site. The patents broadly include TriKE®s that target the CD16 receptor, which includes the more potent camelid nanobody sequence, an IL-15 activating domain, and any targeting domain.
| 6 |
University of Minnesota
2021 Scientific Research Agreement
Effective June 16, 2021, the Company entered into a scientific research agreement with the Regents of the University of Minnesota, expiring on June 30, 2023. Payments totaling approximately $2.1 million are due over the life of the agreement. The purpose of the agreement is for the Regents of the University of Minnesota to continue work with the Company with three major goals in mind: (1) support the Company’s TriKE® product development and GMP manufacturing efforts; (2) TriKE® pharmacokinetics optimization in humans; and (3) investigation of the patient’s native NK cell population based on insights obtained from the analysis of the human data generated during our GTB-3550 clinical trial. The major deliverables proposed are: (1) creation of IND enabling data for TriKE® constructs in support of our product development and GMP manufacturing efforts; (2) TriKE® platform drug delivery changes to allow transition to alternative drug delivery means and extended PK in humans; and (3) gain an increased understanding of changes in the patient’s native NK cell population as a result of TriKE® therapy. Most studies will use TriKE® DNA/amino acid sequences created by the Company under existing licensing terms.
The Company recorded an expense classified as research and development of approximately $0 and $192,000, pursuant to the 2021 Scientific Research Agreement, for the years ended December 31, 2024 and 2023, respectively.
As of December 31, 2024 the Company’s commitments in relation to unbilled and unaccrued amounts from the University of Minnesota pursuant to the 2021 Scientific Research Agreement for services that have not yet been rendered as of December 31, 2024, amounted to $0.
2023 Sponsored Research Agreement
On May 20, 2024, the Company entered into a sponsored research agreement with the Regents of the University of Minnesota (the “2023 Sponsored Research Agreement”), effective July 1, 2023, and expiring on July 1, 2025. Payments totaling approximately $1.7 million are due over the life of the agreement. The purpose of the agreement is for the Regents of the University of Minnesota to continue work with the Company with three major goals in mind: (1) support the Company’s TriKE® product development and commercial GMP manufacturing efforts; (2) TriKE® pharmacokinetics optimization in humans and investigation of effects of altering the route of administration; and (3) research and development of TriKE® platform. The major deliverables proposed are: (1) creation of IND enabling data for TriKE® constructs in support of the Company’s product development and commercial GMP manufacturing efforts outside of the University of Minnesota; (2) TriKE® platform drug delivery changes to allow transition from intravenous (IV) continuous infusion to alternative drug delivery administration (IV bolus, intraperitoneal [IP], subcutaneous [SQ]) and extended PK in humans and gain an increased understanding of changes in the patient’s native NK cell population as a result of alteration of TriKE® administration; and (3) research and development of TriKE® platform combination with other FDA approved (or soon to be approved) therapeutics and alterations to TriKE® platform through formation of immune complexes. Most studies will use TriKE® DNA/amino acid sequences created by the Company under existing licensing terms.
The Company recorded an expense classified as research and development of approximately $1,078,000 and $0, pursuant to the 2023 Sponsored Research Agreement, for the years ended December 31, 2024 and 2023, respectively.
As of December 31, 2024 the Company’s commitments in relation to unbilled and unaccrued amounts from the University of Minnesota pursuant to the 2023 Sponsored Research Agreement for services that have not yet been rendered as of December 31, 2024, amounted to approximately $647,000.
2016 Exclusive Patent License Agreement
Effective July 18, 2016, the Company entered into an exclusive patent license agreement with the Regents of the University of Minnesota (as amended, the “2016 Exclusive Patent License Agreement”), to further develop and commercialize cancer therapies using TriKE® technology developed by researchers at the University of Minnesota to target NK cells to cancer. Under the terms of the agreement, the Company receives exclusive rights to conduct research and to develop, make, use, sell, and import TriKE® technology worldwide for the treatment of any disease, state, or condition in humans. The Company is responsible for obtaining all permits, licenses, authorizations, registrations, and regulatory approvals required or granted by any governmental authority anywhere in the world that is responsible for the regulation of products such as the TriKE® technology, including without limitation the FDA and the European Agency for the Evaluation of Medicinal Products in the European Union. The agreement requires an upfront payment of $200,000, and license maintenance fees of $200,000 for years 2017 through 2020, and $100,000 per year beginning in year 2021 and each year thereafter. The agreement also includes 4% royalty fees on the net sales of licensed products, not to exceed 6% under subsequent license agreements or amendments to this agreement, and minimum royalty payments due upon the commencement of commercial sales of licensed product is $250,000 beginning in 2022, $2 million beginning in 2025, and $5 million beginning in 2027 throughout the remainder of the term. The agreement also includes numerous performance milestone payments including clinical development milestone payments totaling $3.1 million, and one-time sales milestone payments of $1 million upon reaching $250 million in cumulative gross sales, and $5 million upon reaching $500 million in cumulative gross sales of licensed products.
| 7 |
Effective May 13, 2024, the Company entered into an amended and restated exclusive patent license agreement with the Regents of the University of Minnesota. The amendment requires an upfront payment of $145,000 and amends the license maintenance fees to $50,000 in 2025, and $100,000 per year beginning in year 2026 and each year thereafter. The amendment also includes 1% to 5% royalty fees on the net sales of licensed products, not to exceed 6% under subsequent license agreements or amendments, and minimum royalty payments due upon the commencement of commercial sales of licensed product is $250,000 in year one, $2 million in years two through five, and $5 million in year six throughout the remainder of the term. The amendment also includes numerous performance milestone payments including clinical development milestone payments totaling $3.1 million, and one-time sales milestone, and one-time sales milestone payments of $1 million upon reaching $250 million in cumulative gross sales, and $5 million upon reaching $500 million in cumulative gross sales of licensed products.
The Company recorded an expense classified as research and development of $145,000 and $0, pursuant to the 2016 Exclusive Patent License Agreement, for the years ended December 31, 2024 and 2023, respectively.
2021 Exclusive License Agreement
Effective March 26, 2021, the Company entered into an exclusive license agreement with the Regents of the University of Minnesota (the “2021 Exclusive Patent License Agreement”), specific to the B7H3 targeted TriKE®. The agreement requires an upfront payment of $20,000, and license maintenance fees of $5,000 per year beginning in year 2022 and each year thereafter. The agreement also includes 2.5% to 5% royalty fees on the net sales of licensed products, and minimum royalty payments due upon the commencement of commercial sales of licensed product is $250,000 in year one though four, and $2 million beginning in year five and throughout the remainder of the term. The agreement also includes numerous performance milestone payments including clinical development milestone payments totaling $3.1 million, and one-time sales milestone payments of $1 million upon reaching $250 million in cumulative gross sales, and $5 million upon reaching $500 million in cumulative gross sales of licensed products. There is no double payment intended; if one of the milestone payments has been paid under the 2016 restated exclusive patent license agreement no further payment is due for the corresponding milestone.
The Company did not incur any expenses pursuant to the 2021 Exclusive License Agreement, for years ended December 31, 2024 and 2023, respectively.
Corporate History and Structure
The corporate predecessor of GT Biopharma, Inc, Diagnostic Data, Inc., was incorporated in the state of California in 1965. Diagnostic Data, Inc. changed its incorporation to the state of Delaware on December 21, 1972 and changed its name to DDI Pharmaceuticals, Inc. on March 11, 1985. On September 7, 1994, DDI Pharmaceuticals, Inc. merged with International BioClinical, Inc. and Bioxytech S.A. and changed its name to OXIS International, Inc. On July 17, 2017, OXIS International, Inc. changed its name to GT Biopharma, Inc.
Throughout this Annual Report on Form 10-K, the terms “GTBP,” “we,” “us,” “our,” “the Company” and “our Company” refer to GT Biopharma, Inc.
The GT Biopharma logo, TriKE®, and other trademarks or service marks of GT Biopharma, Inc. appearing in this quarterly report are the property of the Company. This Annual Report on Form 10-K also contains registered marks, trademarks and trade names of other companies. All other trademarks, registered marks and trade names appearing herein are the property of their respective holders.
The Company is a clinical stage biopharmaceutical company focused on the development and commercialization of novel immune-oncology products based on our proprietary Tri-specific Killer Engager (TriKE®), and Tetra-specific Killer Engager (Dual Targeting TriKE®) platforms. The Company’s TriKE® and Dual Targeting TriKE® platforms generate proprietary therapeutics designed to harness and enhance the cancer killing abilities of a patient’s own natural killer cells (NK cells).
Common Stock (February 2024 Reverse Stock-Split)
On February 2, 2024, the Company effectuated a reverse stock-split of its common stock, par value $0.001 per share, at a ratio of 1 for 30. The Company’s common stock began trading on a reverse stock-split-adjusted basis on The Nasdaq Capital Market on February 5, 2024 under the existing trading symbol “GTBP.”
As a result of the reverse stock-split, every thirty (30) shares of issued and outstanding common stock were automatically combined into one issued and outstanding share of common stock, without any change in the par value per share. No fractional shares will be issued in connection with the reverse stock-split. Stockholders who otherwise would be entitled to receive fractional shares of common stock will be entitled to receive their pro-rata portion of the net proceeds obtained from the aggregation and sale by the exchange agent of the fractional shares resulting from the reverse stock-split (reduced by any customary brokerage fees, commission and other expenses). The reverse stock-split reduced the number of shares of common stock outstanding on the effective date of the reverse stock-split from 41,419,000 shares to 1,380,633 shares, subject to minor adjustments due to the treatment of fractional shares. The number of authorized shares of common stock remains unchanged at 250,000,000 shares.
Proportionate adjustments have been made to the per share exercise price and the number of shares of common stock that may be purchased upon exercise of outstanding stock options and warrants for the Company’s common stock, and to the number of shares of common stock reserved for future issuance pursuant to the Company’s 2022 Omnibus Incentive Plan.
All share and per share information within this report have been adjusted to retroactively reflect the reverse stock-split as of the earliest period presented.
| 8 |
Employees and Human Capital Resources
At the date of this Annual Report, we have 1 full-time employee and numerous consultants to carry on our operations. Many of our activities are outsourced to consultants who provide services to us on a project basis. As business activities require and capital resources permit, we will hire additional employees and consultants to fulfill our Company’s needs.
Available Information
We post our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K, and any amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act, free of charge, on the Investors section of our public website (www.gtbiopharma.com) as soon as reasonably practicable after we electronically file such material with, or furnish it to, the SEC . In addition, you can read our SEC filings over the Internet at the SEC’s website at www.sec.gov. The contents of these websites are not incorporated into this annual report on Form 10-K. Further, our references to the URLs for these websites are intended to be inactive textual references only.
ITEM 1A. RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below in addition to the other information contained in this Annual Report on Form 10-K before deciding whether to invest in shares of our common stock. If any of the following risks actually occur, our business, financial condition or operating results could be harmed. In that case, the trading price of our common stock could decline and you may lose part or all of your investment. In the opinion of management, the risks discussed below represent the material risks known to the company. Additional risks and uncertainties not currently known to us or that we currently deem immaterial may also impair our business, financial condition and operating results and adversely affect the market price of our common stock.
Risks Related to Our Business
Our financial condition raises substantial doubt as to our ability to continue as a going concern.
As of December 31, 2024, we had approximately $4.0 million in cash and cash equivalents and restricted cash, and a working capital deficit of $1.7 million, and we have incurred and expect to continue to incur significant costs in pursuit of our drug candidates. For the year ended December 31, 2024, we recorded a net loss of approximately $13.2 million and used cash in operations of approximately $12.9 million. Our financial statements for the year ended December 31, 2024 have been prepared assuming that we will continue to operate as a going concern, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. To date, we have not generated substantial product revenues from our activities and have incurred substantial operating losses. We expect that we will continue to generate substantial operating losses for the foreseeable future until we complete development and approval of our product candidates. We will continue to fund our operations primarily through utilization of our current financial resources and additional raises of capital.
These conditions raise substantial doubt about our ability to continue as a going concern. The Company has evaluated the significance of the uncertainty regarding the Company’s financial condition in relation to its ability to meet its obligations, which has raised substantial doubt about the Company’s ability to continue as a going concern. While it is very difficult to estimate the Company’s future liquidity requirements, the Company believes if it is unable to obtain additional financing, existing cash resources will not be sufficient to enable it to fund the anticipated level of operations through one year from the date the accompanying financial statements are issued. There can be no assurances that the Company will be able to secure additional financing on acceptable terms. In the event the Company does not secure additional financing, the Company will be forced to delay, reduce, or eliminate some or all of its discretionary spending, which could adversely affect the Company’s business prospects, ability to meet long-term liquidity needs and the ability to continue operations.
Our business is at an early stage of development and we may not develop therapeutic products that can be commercialized.
Our business is at an early stage of development. We do not have immune-oncology products in late-stage clinical trials. We are still in the early stages of identifying and conducting research on potential therapeutic products. Our potential therapeutic products will require significant research and development and pre-clinical and clinical testing prior to regulatory approval in the United States and other countries. We may not be able to obtain regulatory approvals, enter clinical trials for any of our product candidates, or commercialize any products. Our product candidates may prove to have undesirable and unintended side effects or other characteristics adversely affecting their safety, efficacy or cost effectiveness that could prevent or limit their use. Any product using any of our technology may fail to provide the intended therapeutic benefits or achieve therapeutic benefits equal to or better than the standard of treatment at the time of testing or production.
| 9 |
We have a history of operating losses and we expect to continue to incur losses for the foreseeable future and we may never generate revenue or achieve profitability.
During the year ended December 31, 2024, the Company reported a net loss of $13.2 million and as of December 31, 2024 and had an accumulated deficit of approximately $695 million. We have not generated any revenue to date and are not profitable, and have incurred losses in each year since our inception. We do not expect to generate any product sales or royalty revenues for the foreseeable future. We expect to incur significant additional operating losses for the foreseeable future as we expand research and development and clinical trial efforts.
Our ability to achieve long-term profitability is dependent upon obtaining regulatory approvals for our products and successfully commercializing our products alone or with third parties. However, our operations may not be profitable even if any of our products under development are successfully developed and produced and thereafter commercialized. Even if we achieve profitability in the future, we may not be able to sustain profitability in subsequent periods.
Even if we succeed in commercializing one or more of our product candidates, we expect to continue to incur substantial research and development and other expenditures to develop and market additional product candidates. The size of our future net losses will depend, in part, on the rate of future growth of our expenses and our ability to generate revenue. Our prior losses and expected future losses have had and will continue to have an adverse effect on our stockholders’ equity and working capital.
We will need additional capital to conduct our operations and develop our products, and our ability to obtain the necessary funding is uncertain.
We have used a significant amount of cash since inception to finance the continued development and testing of our product candidates, and we expect to need substantial additional capital resources to develop our product candidates going forward and launch and commercialize any product candidates for which we receive regulatory approval.
We may not be successful in generating and/or maintaining operating cash flow, and the timing of our capital expenditures and other expenditures may not result in cash sufficient to sustain our operations through the commercialization of our product candidates. If financing is not sufficient and additional financing is not available or available only on terms that are detrimental to our long-term survival, it could have a material adverse effect on our ability to continue to function. The timing and degree of any future capital requirements will depend on many factors, including:
| ● | the accuracy of the assumptions underlying our estimates for capital needs in 2025 and beyond; | |
| ● | scientific and clinical progress in our research and development programs; | |
| ● | the magnitude and scope of our research and development programs and our ability to establish, enforce and maintain strategic arrangements for research, development, clinical testing, manufacturing and marketing; | |
| ● | our progress with pre-clinical development and clinical trials; | |
| ● | the time and costs involved in obtaining regulatory approvals; | |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; and | |
| ● | the number and type of product candidates that we pursue. |
Additional financing through strategic collaborations, public or private equity or debt financings or other financing sources may not be available on acceptable terms, or at all. Additional equity financing could result in significant dilution to our stockholders, and any debt financings will likely involve covenants restricting our business activities. Further, if we obtain additional funds through arrangements with collaborative partners, these arrangements may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise seek to develop and commercialize on our own.
If sufficient capital is not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or product development initiatives, any of which could have a material adverse effect on our financial condition or business prospects.
| 10 |
Our research and development costs could exceed our projections requiring us to significantly modify our planned operations.
The actual cost of our research and development programs could differ significantly from our current projections if we change our planned development process. In the event that actual costs of our clinical program, or any of our other ongoing research activities, are significantly higher than our current estimates, we may be required to significantly modify our planned level of operations.
The successful development of any product candidate is highly uncertain. It is difficult to reasonably estimate or know the nature, timing and costs of the efforts necessary to complete the development of, or the period in which material net cash inflows are expected to commence from any product candidate, due to the numerous risks and uncertainties associated with developing drugs. Any failure to complete any stage of the development of products in a timely manner could have a material adverse effect on our operations, financial position and liquidity.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, stockholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our common stock.
Effective internal control over financial reporting is necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation, could cause us to fail to meet our reporting obligations. Ineffective internal control could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our common stock.
As defined in Regulation 12b-2 under the Securities Exchange Act of 1934, or the Exchange Act, a “material weakness” is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented, or detected on a timely basis.
We have taken measures to mitigate potential issues and have implemented a functional system of internal controls over financial reporting. However, such controls may become inadequate due to changes in conditions, or the degree of compliance with such policies or procedures may deteriorate, which could result in the discovery of material weaknesses and deficiencies. In any event, the process of determining whether our existing internal control over financial reporting is compliant with Section 404 of the Sarbanes-Oxley Act, or Section 404, and sufficiently effective requires the investment of substantial time and resources, including by certain members of our senior management.
We are required, pursuant to Section 404, to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting. However, for as long as we are a “smaller reporting company,” our independent registered public accounting firm will not be required to attest to the effectiveness of our internal control over financial reporting pursuant to Section 404. While we could be a smaller reporting company for an indefinite amount of time, and thus relieved of the above-mentioned attestation requirement, an independent assessment of the effectiveness of our internal control over financial reporting could detect problems that our management’s assessment might not. Such undetected material weaknesses in our internal control over financial reporting could lead to financial statement restatements and require us to incur the expense of remediation.
Our intellectual property may be compromised.
Part of our value going forward depends on the intellectual property rights that we have been and are acquiring. There may have been many persons involved in the development of our intellectual property, and we may not be successful in obtaining the necessary rights from all of them. It is possible that in the future, third parties may challenge our intellectual property rights. We may not be successful in protecting our intellectual property rights. In either event, we may lose the value of our intellectual property, and if so, our business prospects may suffer.
If our efforts to protect the proprietary nature of the intellectual property related to our technologies are not adequate, we may not be able to compete effectively in our market and our business would be harmed.
We rely upon a combination of patents, trade secret protection and confidentiality agreements to protect the intellectual property related to our technologies. Any disclosure to or misappropriation by third parties of our trade secret or other confidential information could enable competitors to quickly duplicate or surpass our technological achievements, thus eroding any competitive advantage we may derive from this information.
The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications we own or license may fail to result in issued patents in the United States or in foreign countries. Third parties may challenge the validity, enforceability or scope of any issued patents we own or license or any applications that may be issued as patents in the future, which may result in those patents being narrowed, invalidated or held unenforceable. Even if they are unchallenged, our patents and patent applications may not adequately protect our intellectual property or prevent others from developing similar products that do not fall within the scope of our patents. If the breadth or strength of protection provided by the patents we hold or pursue is threatened, our ability to commercialize any product candidates with technology protected by those patents could be threatened. Further, if we encounter delays in our clinical trials, the time during which we would have patent protection for any covered product candidates that obtain regulatory approval would be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, we cannot be certain at the time of filing that we are the first to file any patent application related to our product candidates.
| 11 |
In addition to the protection afforded by patents, we seek to rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our discovery platform and drug development processes that involve proprietary know-how, information or technology that is not covered by patents or not amenable to patent protection. Although we require all of our employees and certain consultants and advisors to assign inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, our trade secrets and other proprietary information may be disclosed or competitors may otherwise gain access to such information or independently develop substantially equivalent information. Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant difficulty in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the trade secret intellectual property related to our technologies to third parties, we may not be able to establish or maintain the competitive advantage that we believe is provided by such intellectual property, which could materially adversely affect our market position and business and operational results.
Claims that we infringe the intellectual property rights of others may prevent or delay our drug discovery and development efforts.
Our research, development and commercialization activities, as well as any product candidates or products resulting from those activities, may infringe or be accused of infringing a patent or other form of intellectual property under which we do not hold a license or other rights. Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents of which we are currently unaware, with claims that cover the use or manufacture of our product candidates or the practice of our related methods. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes one or more claims of these patents. If our activities or product candidates infringe the patents or other intellectual property rights of third parties, the holders of such intellectual property rights may be able to block our ability to commercialize such product candidates or practice our methods unless we obtain a license under the intellectual property rights or until any applicable patents expire or are determined to be invalid or unenforceable.
Defense of any intellectual property infringement claims against us, regardless of their merit, would involve substantial litigation expense and would be a significant diversion of employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, obtain one or more licenses from third parties, limit our business to avoid the infringing activities, pay royalties and/or redesign our infringing product candidates or methods, any or all of which may be impossible or require substantial time and monetary expenditure. Further, if we were to seek a license from the third-party holder of any applicable intellectual property rights, we may not be able to obtain the applicable license rights when needed or on commercially reasonable terms, or at all. The occurrence of any of the above events could prevent us from continuing to develop and commercialize one or more of our product candidates and our business could materially suffer.
We may desire, or be forced, to seek additional licenses to use intellectual property owned by third parties, and such licenses may not be available on commercially reasonable terms or at all.
A third party may hold intellectual property, including patent rights, that are important or necessary to the development of our product candidates, in which case we would need to obtain a license from that third party or develop a different formulation of the product that does not infringe upon the applicable intellectual property, which may not be possible. Additionally, we may identify product candidates that we believe are promising and whose development and other intellectual property rights are held by third parties. In such a case, we may desire to seek a license to pursue the development of those product candidates. Any license that we may desire to obtain or that we may be forced to pursue may not be available when needed on commercially reasonable terms or at all. Any inability to secure a license that we need or desire could have a material adverse effect on our business, financial condition and prospects.
The patent protection covering some of our product candidates may be dependent on third parties, who may not effectively maintain that protection.
While we expect that we will generally seek to gain the right to fully prosecute any patents covering product candidates we may in-license from third-party owners, there may be instances when platform technology patents that cover our product candidates remain controlled by our licensors. If any of our current or future licensing partners that retain the right to prosecute patents covering the product candidates we license from them fail to appropriately maintain that patent protection, we may not be able to prevent competitors from developing and selling competing products or practicing competing methods and our ability to generate revenue from any commercialization of the affected product candidates may suffer.
| 12 |
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe our patents or the patents of our current or potential licensors. To attempt to stop infringement or unauthorized use, we may need to enforce one or more of our patents, which can be expensive and time-consuming and distract management. If we pursue any litigation, a court may decide that a patent of ours or our licensor’s is not valid or is unenforceable, or may refuse to stop the other party from using the relevant technology on the grounds that our patents do not cover the technology in question. Further, the legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, which could reduce the likelihood of success of any infringement proceeding we pursue in any such jurisdiction. An adverse result in any infringement litigation or defense proceedings could put one or more of our patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put our patent applications at risk of not issuing, which could limit our ability to exclude competitors from directly competing with us in the applicable jurisdictions.
Interference proceedings provoked by third parties or brought by the U.S. PTO may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to use it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms, or at all. Litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees.
If we are unsuccessful in obtaining or maintaining patent protection for intellectual property in development, our business and competitive position would be harmed.
We are seeking patent protection for some of our technology and product candidates. Patent prosecution is a challenging process and is not assured of success. If we are unable to secure patent protection for our technology and product candidates, our business may be adversely impacted.
In addition, issued patents and pending international applications require regular maintenance. Failure to maintain our portfolio may result in loss of rights that may adversely impact our intellectual property rights, for example by rendering issued patents unenforceable or by prematurely terminating pending international applications.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We currently, and expect in the future to continue to, seek to protect these trade secrets, in part, by entering into confidentiality agreements with parties who have access to them, such as our employees, collaborators, contract manufacturers, consultants, advisors and other third parties. Despite these efforts, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for any such disclosure. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they disclose the trade secrets, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
If we fail to meet our obligations under our license agreements, we may lose our rights to key technologies on which our business depends.
Our business depends in part on licenses from third parties. These third-party license agreements impose obligations on us, such as payment obligations and obligations to diligently pursue development of commercial products under the licensed patents. If a licensor believes that we have failed to meet our obligations under a license agreement, the licensor could seek to limit or terminate our license rights, which could lead to costly and time-consuming litigation and, potentially, a loss of the licensed rights. During the period of any such litigation, our ability to carry out the development and commercialization of potential products could be significantly and negatively affected. If our license rights were restricted or ultimately lost, our ability to continue our business based on the affected technology platform could be severely adversely affected.
| 13 |
We will have to hire additional employees to carry on our business operations. If we are unable to hire qualified personnel, we may not be able to implement our business strategy.
We currently have one full-time employee and numerous consultants to carry on our operations. Our Interim Chief Executive Officer and Executive Chairman of the Board provides his services through a consulting arrangement. The loss of the services of any of our employees or consultants could delay our product development programs and our research and development efforts. In order to develop our business in accordance with our business strategy, we will have to hire additional qualified personnel, including in the areas of manufacturing, clinical trials management, regulatory affairs, finance, discovery biology, and business development. We will need to raise sufficient funds to hire and retain the necessary employees and consultants.
Moreover, there is intense competition for a limited number of qualified personnel among biopharmaceutical, biotechnology, pharmaceutical and other businesses. Many of the other pharmaceutical companies against which we compete for qualified personnel have greater financial and other resources, different risk profiles, longer histories in the industry and greater ability to provide valuable cash or stock incentives to potential recruits than we do. They also may provide more diverse opportunities and better chances for career advancement. Some of these characteristics may be more appealing to high quality candidates than what we are able to offer as an early-stage company. If we are unable to continue to attract and retain high quality personnel, the rate and success at which we can develop and commercialize product candidates will be limited.
We depend on key personnel for our continued operations and future success, and a loss of certain key personnel could significantly hinder our ability to move forward with our business plan.
Because of the specialized nature of our business, we are highly dependent on our ability to identify, hire, train and retain highly qualified scientific and technical personnel for the research and development activities we conduct or sponsor. The loss of one or more key executive officers, scientific or operational team members would be significantly detrimental to us. In addition, recruiting and retaining qualified scientific personnel to perform research and development work is critical to our success. Our anticipated growth and expansion into areas and activities requiring additional expertise, such as discovery biology, clinical testing, regulatory compliance, manufacturing and compliance, will require the addition of new management personnel and the development of additional expertise by existing management personnel. There is intense competition for qualified personnel in the areas of our present and planned activities. Accordingly, we may not be able to continue to attract and retain the qualified personnel, which would adversely affect the development of our business.
We may be subject to claims by third parties asserting that our employees or we have misappropriated their intellectual property, or claiming ownership of what we regard as our own intellectual property.
In the past, many of our employees were employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees do not use the proprietary information or know-how of others in their work for us, with contractual provisions and other procedures, we may be subject to claims that these employees or we have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such employee’s former employers. Litigation may be necessary to defend against any such claims.
In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with each party who in fact contributes to the development of intellectual property that we regard as our own. Further, the terms of such assignment agreements may be breached and we may not be able to successfully enforce their terms, which may force us to bring claims against third parties, or defend claims they may bring against us, to determine the ownership of intellectual property rights we may regard and treat as our own.
Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, which could cause our business to suffer.
We are exposed to the risk of employee fraud or other misconduct. Misconduct by employees could include intentional failures to comply with regulations of governmental authorities, such as the U.S. Food and Drug Administration (“FDA”) or the European Medicines Agency, or EMA, to provide accurate information to the FDA or EMA, to comply with manufacturing standards we have established, to comply with federal, state and international healthcare fraud and abuse laws and regulations as they may become applicable to our operations, to report financial information or data accurately or to disclose unauthorized activities to us. Employee misconduct could also involve the improper use of information obtained during clinical trials, which could result in regulatory sanctions and serious harm to our reputation. It is not always possible to identify and deter employee misconduct, and the precautions we currently take and the procedures we may establish in the future as our operations and employee base expand to detect and prevent this type of activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure by our employees to comply with such laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business and results of operations, including the imposition of significant fines or other sanctions.
| 14 |
Our reliance on the activities of our non-employee consultants, research institutions and scientific contractors, whose activities are not wholly within our control, may lead to delays in development of our proposed products.
We rely extensively upon and have relationships with scientific consultants at academic and other institutions, some of whom conduct research at our request, and other consultants with expertise in clinical development strategy or other matters. These consultants are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. We have limited control over the activities of these consultants and, except as otherwise required by our collaboration and consulting agreements to the extent they exist, can expect only limited amounts of their time to be dedicated to our activities. These research facilities may have commitments to other commercial and non-commercial entities. We have limited control over the operations of these laboratories and can expect only limited amounts of time to be dedicated to our research goals.
It may take longer to complete our clinical trials than we project, or we may not be able to complete them at all.
For budgeting and planning purposes, we have projected the date for the commencement, continuation and completion of our various clinical trials. However, a number of factors, including scheduling conflicts with participating clinicians and clinical institutions, and difficulties in identifying and enrolling patients who meet trial eligibility criteria, may cause significant delays. We may not commence or complete clinical trials involving any of our products as projected or may not conduct them successfully.
We expect to rely on medical institutions, academic institutions or clinical research organizations to conduct, supervise or monitor some or all aspects of clinical trials involving our products. We will have less control over the timing and other aspects of these clinical trials than if we conducted them entirely on our own. If we fail to commence or complete, or experience delays in, any of our planned clinical trials, our stock price and our ability to conduct our business as currently planned could be harmed.
Clinical drug development is costly, time-consuming and uncertain, and we may suffer setbacks in our clinical development program that could harm our business.
Clinical drug development for our product candidates is costly, time-consuming and uncertain. Our product candidates are in various stages of development and while we expect that clinical trials for these product candidates will continue for several years, such trials may take significantly longer than expected to complete. In addition, we, the FDA, an Institutional Review Board, or IRB, or other regulatory authorities, including state and local agencies and counterpart agencies in foreign countries, may suspend, delay, require modifications to or terminate our clinical trials at any time, for various reasons, including:
| ● | discovery of safety or tolerability concerns, such as serious or unexpected toxicities or side effects or exposure to otherwise unacceptable health risks, with respect to study participants; | |
| ● | lack of effectiveness of any product candidate during clinical trials or the failure of our product candidates to meet specified endpoints; | |
| ● | delays in subject recruitment and enrollment in clinical trials or inability to enroll a sufficient number of patients in clinical trials to ensure adequate statistical ability to detect statistically significant treatment effects; | |
| ● | difficulty in retaining subjects and volunteers in clinical trials; | |
| ● | difficulty in obtaining the IRB’s approval for studies to be conducted at each clinical trial site; | |
| ● | inadequacy of or changes in our manufacturing process or the product formulation or method of delivery; | |
| ● | delays or failure in reaching agreement on acceptable terms in clinical trial contracts or protocols with prospective Contract Research Organizations, (“CROs”), clinical trial sites and other third-party contractors; | |
| ● | inability to add a sufficient number of clinical trial sites; | |
| ● | uncertainty regarding proper formulation and dosing; | |
| ● | failure by us, our employees, our CROs or their employees or other third-party contractors to comply with contractual and applicable regulatory requirements or to perform their services in a timely or acceptable manner; | |
| ● | scheduling conflicts with participating clinicians and clinical institutions; | |
| ● | failure to design appropriate clinical trial protocols; | |
| ● | inability or unwillingness of medical investigators to follow our clinical protocols; | |
| ● | difficulty in maintaining contact with subjects during or after treatment, which may result in incomplete data; or | |
| ● | changes in applicable laws, regulations and regulatory policies. |
| 15 |
If we experience delays or difficulties in the enrollment of patients in clinical trials, those clinical trials could take longer than expected to complete and our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for our product candidates if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA, or similar regulatory authorities outside the United States. In particular, because we are focused on patients with molecularly defined cancers, our pool of suitable patients may be smaller and more selective and our ability to enroll a sufficient number of suitable patients may be limited or take longer than anticipated. In addition, some of our competitors have ongoing clinical trials for product candidates that treat the same indications as our product candidates, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates.
Patient enrollment for any of our clinical trials may also be affected by other factors, including without limitation:
| ● | the severity of the disease under investigation; | |
| ● | the frequency of the molecular alteration we are seeking to target in the applicable trial; | |
| ● | the eligibility criteria for the study in question; | |
| ● | the perceived risks and benefits of the product candidate under study; | |
| ● | the extent of the efforts to facilitate timely enrollment in clinical trials; | |
| ● | the patient referral practices of physicians; | |
| ● | the ability to monitor patients adequately during and after treatment; and | |
| ● | the proximity and availability of clinical trial sites for prospective patients. |
Our inability to enroll a sufficient number of patients for our clinical trials would result in significant delays and could require us to abandon one or more clinical trials altogether. Enrollment delays in our clinical trials may result in increased development costs for our product candidates, and we may not have or be able to obtain sufficient cash to fund such increased costs when needed, which could result in the further delay or termination of the trial.
Consistent with our general product development strategy, we intend to design future trials for our product candidates to include some patients with the applicable clinical characteristics, stage of therapy, molecular alterations, biomarkers, and/or cell surface antigens that determine therapeutic options, or are indicators of the disease, with a view to assessing possible early evidence of potential therapeutic effect. If we are unable to locate and include such patients in those trials, then our ability to make those early assessments and to seek participation in FDA expedited review and approval programs, including breakthrough therapy and fast track designation, or otherwise to seek to accelerate clinical development and regulatory timelines, could be compromised.
We have limited clinical testing and regulatory capabilities, and human clinical trials are subject to extensive regulatory requirements, which are very expensive, time-consuming and difficult to design and implement. Our products may fail to achieve necessary safety and efficacy endpoints during clinical trials, which may limit our ability to generate revenues from therapeutic products.
We cannot assure that we will be able to invest or develop resources for clinical trials successfully or as expediently as necessary. In particular, human clinical trials can be very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The clinical trial process is time consuming. We estimate that clinical trials of our product candidates will take at least several years to complete. Furthermore, failure can occur at any stage of the trials, and we could encounter problems that cause us to abandon or repeat clinical trials. The commencement and completion of clinical trials may be affected by several factors, including:
| ● | unforeseen safety issues; | |
| ● | determination of dosing issues; | |
| ● | inability to demonstrate effectiveness during clinical trials; | |
| ● | slower than expected rates of patient recruitment; | |
| ● | inability to monitor patients adequately during or after treatment; and | |
| ● | inability or unwillingness of medical investigators to follow our clinical protocols. |
| 16 |
In addition, we or the FDA, may suspend our clinical trials at any time if it appears that we are exposing participants to unacceptable health risks or if the FDA finds deficiencies in our investigational new drug application, or IND, submissions or the conduct of these trials.
We are subject to extensive regulation, which can be costly and time consuming and can subject us to unanticipated delays. even if we obtain regulatory approval for some of our products, those products may still face regulatory difficulties.
All of our potential products, processing and manufacturing activities, are subject to comprehensive regulation by the FDA in the United States and by comparable authorities in other countries. The process of obtaining FDA and other required regulatory approvals, including foreign approvals, is expensive and often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. In addition, regulatory agencies may lack experience with our technologies and products, which may lengthen the regulatory review process, increase our development costs and delay or prevent their commercialization.
If we violate regulatory requirements at any stage, whether before or after we obtain marketing approval, the FDA may take enforcement action(s) against us, which could include issuing a warning or untitled letter, placing a clinical hold on an ongoing clinical trial, product seizure, enjoining our operations, refusal to consider our applications for pre-market approval, refusal of an investigational new drug application, fines, or even civil or criminal liability, any of which could materially harm our reputation and financial results. Additionally, we may not be able to obtain the labeling claims necessary or desirable for the promotion of our products. We may also be required to undertake post marketing trials to provide additional evidence of safety and effectiveness. In addition, if we or others identify side effects after any of our adoptive therapies are on the market, or if manufacturing problems occur, regulators may withdraw their approval and reformulations, additional clinical trials, changes in labeling of our products, and additional marketing applications may be required.
Any of the following factors, among others, could cause regulatory approval for our product candidates to be delayed, limited or denied:
| ● | the product candidates require significant clinical testing to demonstrate safety and effectiveness before applications for marketing approval can be filed with the FDA and other regulatory authorities; | |
| ● | data obtained from pre-clinical and nonclinical animal testing and clinical trials can be interpreted in different ways, and regulatory authorities may not agree with our respective interpretations or may require us to conduct additional testing; | |
| ● | negative or inconclusive results or the occurrence of serious or unexpected adverse events during a clinical trial could cause us to delay or terminate development efforts for a product candidate; and/or | |
| ● | FDA and other regulatory authorities may require expansion of the size and scope of the clinical trials. |
Any difficulties or failures that we encounter in securing regulatory approval for our product candidates would likely have a substantial adverse impact on our ability to generate product sales, and could make a search for a collaborative partner more difficult.
Obtaining regulatory approval even after clinical trials that are believed to be successful is an uncertain process.
Even if we complete our planned clinical trials and believe the results were successful, obtaining regulatory approval is a lengthy, expensive and uncertain process, and the FDA or other regulatory agencies may delay, limit or deny approval of any of our applications for pre-market approval for many reasons, including:
| ● | we may not be able to demonstrate to the FDA’s satisfaction that our product candidates are safe and effective for any indication; | |
| ● | the results of clinical trials may not meet the level of statistical significance or clinical significance required by the FDA for approval; | |
| ● | the FDA may disagree with the number, design, size, conduct or implementation of our clinical trials; | |
| ● | the FDA may not find the data from pre-clinical studies and clinical trials sufficient to demonstrate that the clinical and other benefits of our product candidates outweigh their safety risks; |
| 17 |
| ● | the FDA may disagree with our interpretation of data from pre-clinical studies or clinical trials, or may not accept data generated at our clinical trial sites; | |
| ● | the data collected from pre-clinical studies and clinical trials of our product candidates may not be sufficient to support the submission of applications for regulatory approval; | |
| ● | the FDA may have difficulties scheduling an advisory committee meeting in a timely manner, or the advisory committee may recommend against approval of our application or may recommend that the FDA require, as a condition of approval, additional pre-clinical studies or clinical trials, limitations on approved labeling, or distribution and use restrictions; | |
| ● | the FDA may require development of a risk evaluation and mitigation strategy as a condition of approval; | |
| ● | the FDA may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we enter into agreements for clinical and commercial supplies; | |
| ● | the FDA may change their approval policies or adopt new regulations that adversely affect our applications for pre-market approval; and | |
| ● | the FDA may require simultaneous approval for both adults and for children and adolescents delaying needed approvals, or we may have successful clinical trial results for adults but not children and adolescents, or vice versa. |
Before we can submit an application for regulatory approval in the United States, we must conduct a pivotal, registrational trial. We will also need to agree on a protocol with the FDA for a clinical trial before commencing the trial. Registrational clinical trials frequently produce unsatisfactory results even though prior clinical trials were successful. Therefore, even if the results of our early phase trials are successful, the results of the additional trials that we conduct may or may not be successful. Further, our product candidates may not be approved even if they achieve their primary endpoints in registrational clinical trials. The FDA or other foreign regulatory authorities may disagree with our trial design and our interpretation of data from preclinical studies and clinical trials. Any of these regulatory authorities may change requirements for the approval of a product candidate even after reviewing and providing comments or advice on a protocol for a clinical trial. The FDA or other regulatory agencies may require that we conduct additional clinical, nonclinical, manufacturing validation or drug product quality studies and submit those data before considering or reconsidering the application. Depending on the extent of these or any other studies, approval of any applications that we submit may be delayed by several years, or may require us to expend more resources than we have available. It is also possible that additional studies, if performed and completed, may not be considered sufficient by the FDA or other regulatory agencies.
In addition, the FDA or other regulatory agencies may also approve a product candidate for fewer or more limited indications than we request, may impose significant limitations related to use restrictions for certain age groups, warnings, precautions or contraindications or may grant approval contingent on the performance of costly post-marketing clinical trials or risk mitigation requirements.
We will continue to be subject to extensive FDA regulation following any product approvals, and if we fail to comply with these regulations, we may suffer a significant setback in our business.
Even if we are successful in obtaining regulatory approval of our product candidates, we will continue to be subject to the requirements of and review by, the FDA and comparable regulatory authorities in the areas of manufacturing processes, post-approval clinical data, adverse event reporting, labeling, advertising and promotional activities, among other things. In addition, any marketing approval we receive may be limited in terms of the approved product indication or require costly post-marketing testing and surveillance. Discovery after approval of previously unknown problems with a product, manufacturer or manufacturing process, or a failure to comply with regulatory requirements, may result in enforcement actions such as:
| ● | warning letters or other actions requiring changes in product manufacturing processes or restrictions on product marketing or distribution; | |
| ● | product recalls or seizures or the temporary or permanent withdrawal of a product from the market; | |
| ● | suspending any ongoing clinical trials; | |
| ● | temporary or permanent injunctions against our production operations; | |
| ● | refusal of our applications for pre-market approval or an investigational new drug application; and | |
| ● | fines, restitution or disgorgement of profits or revenue, the imposition of civil penalties or criminal prosecution. |
| 18 |
The occurrence of any of these actions would likely cause a material adverse effect on our business, financial condition and results of operations.
Many of our business practices are subject to scrutiny and potential investigation by regulatory and government enforcement authorities, as well as to lawsuits brought by private citizens under federal and state laws. We could become subject to investigations, and our failure to comply with applicable law or an adverse decision in lawsuits may result in adverse consequences to us. If we fail to comply with U.S. healthcare laws, we could face substantial penalties and financial exposure, and our business, operations and financial condition could be adversely affected.
While payment is not yet available from third-party payors (government or commercial) for our product, our goal is to obtain such coverage as soon as possible after product approval and commercial launch in the U.S. If this occurs, the availability of such payment would mean that many healthcare laws would place limitations and requirements on the manner in which we conduct our business (including our sales and promotional activities and interactions with healthcare professionals and facilities) and could result in liability and exposure to us. In some instances, our interactions with healthcare professionals and facilities that occurred prior to commercialization could have implications at a later date. The laws that may affect our ability to operate include, among others: (i) the federal healthcare programs Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, receiving, offering or paying remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs such as Medicare or Medicaid; (ii) federal false claims laws which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us under theories of “implied certification” where the government and qui tam relators may allege that device companies are liable where a product that was paid for by the government in whole or in part was promoted “off-label,” lacked necessary approval, or failed to comply with good manufacturing practices or other laws; (iii) transparency laws and related reporting and/or disclosures such as the Sunshine Act; and/or (iv) state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, many of which differ from their federal counterparts in significant ways, thus complicating compliance efforts.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, exclusion from participation in government healthcare programs, damages, fines and the curtailment or restructuring of our operations. Any penalties, damages, fines, curtailment or restructuring of our operations could adversely affect our ability to operate our business and our financial results. The risk of our being found in violation of these laws is increased by the fact that their provisions are open to a variety of evolving interpretations and enforcement discretion. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business.
Both federal and state government agencies have heightened civil and criminal enforcement efforts. There are numerous ongoing investigations of healthcare pharmaceutical companies and others in the healthcare space, as well as their executives and managers. In addition, amendments to the Federal False Claims Act, have made it easier for private parties to bring qui tam (whistleblower) lawsuits against companies under which the whistleblower may be entitled to receive a percentage of any money paid to the government. In addition, the Affordable Care Act amended the federal civil False Claims Act to provide that a claim that includes items or services resulting from a violation of the federal anti-kickback statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Penalties include substantial fines for each false claim, plus three times the amount of damages that the federal government sustained because of the act of that person or entity and/or exclusion from the Medicare program. In addition, a majority of states have adopted similar state whistleblower and false-claims provision. There can be no assurance that our activities will not come under the scrutiny of regulators and other government authorities or that our practices will not be found to violate applicable laws, rules and regulations or prompt lawsuits by private citizen “relators” under federal or state false claims laws. Any future investigations of our business or executives, or enforcement action or prosecution, could cause us to incur substantial costs, and result in significant liabilities or penalties, as well as damage to our reputation.
Laws impacting the U.S. healthcare system are subject to a great deal of uncertainty, which may result in adverse consequences to our business.
There have been a number of legislative and regulatory proposals to change the healthcare system, reduce the costs of healthcare and change medical reimbursement policies. Doctors, clinics, hospitals and other users of our products may decline to purchase our products to the extent there is uncertainty regarding coverage from government or commercial payors. Further proposed legislation, regulation and policy changes affecting third-party reimbursement are likely. Among other things, Congress has in the past proposed changes to and the repeal of the Patient Protection and Affordable Care and Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the “Affordable Care Act”), and lawsuits have been brought challenging aspects of the law at various points. There have been repeated recent attempts by Congress to repeal or replace the Affordable Care Act. At this time, it remains unclear whether there will be any changes made to or any repeal or replacement of the Affordable Care Act, with respect to certain of its provisions or in its entirety. We are unable to predict what legislation or regulation, if any, relating to the health care industry or third-party coverage and reimbursement may be enacted in the future at the state or federal level, or what effect such legislation or regulation may have on us. Denial of coverage and reimbursement of our products, or the revocation or changes to coverage and reimbursement policies, could have a material adverse effect on our business, results of operations and financial condition.
| 19 |
We may not be successful in our efforts to build a pipeline of product candidates.
A key element of our strategy is to use and expand our product platform to build a pipeline of product candidates and progress those product candidates through clinical development for the treatment of a variety of different types of cancer. Even if we are successful in building a product pipeline, the potential product candidates that we identify may not be suitable for clinical development for a number of reasons, including causing harmful side effects or demonstrating other characteristics that indicate a low likelihood of receiving marketing approval or achieving market acceptance. If our methods of identifying potential product candidates fail to produce a pipeline of potentially viable product candidates, then our success as a business will be dependent on the success of fewer potential product candidates, which introduces risks to our business model and potential limitations to any success we may achieve.
Our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following marketing approval, if any.
Additionally, if one or more of our product candidates receives marketing approval, and we or others later identify undesirable side effects caused by such products, a number of potentially significant negative consequences could result, including:
| ● | regulatory authorities may withdraw approvals of such product; | |
| ● | regulatory authorities may require additional warnings on the product’s label; | |
| ● | we may be required to create a medication guide for distribution to patients that outlines the risks of such side effects; | |
| ● | we could be sued and held liable for harm caused to patients; and | |
| ● | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of the particular product candidate, if approved, and could significantly harm our business, results of operations and prospects.
We may expend our limited resources to pursue a particular product candidate or indication that does not produce any commercially viable products and may fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we must focus our efforts on particular research programs and product candidates for specific indications. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. Further, our resource allocation decisions may result in our use of funds for research and development programs and product candidates for specific indications that may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. Any such failure to improperly assess potential product candidates could result in missed opportunities and/or our focus on product candidates with low market potential, which would harm our business and financial condition.
Our products may be expensive to manufacture, and they may not be profitable if we are unable to control the costs to manufacture them.
Our products may be significantly more expensive to manufacture than we expect or than other therapeutic products currently on the market today. We hope to substantially reduce manufacturing costs through process improvements, development of new methods, increases in manufacturing scale and outsourcing to experienced manufacturers. If we are not able to make these, or other improvements, and depending on the pricing of the product, our profit margins may be significantly less than that of other therapeutic products on the market today. In addition, we may not be able to charge a high enough price for any product we develop, even if they are safe and effective, to make a profit. If we are unable to realize significant profits from our potential product candidates, our business would be materially harmed.
The interruption of the flow of raw materials could disrupt the supply chain and our ability to manufacture our products.
We depend on our outsourced manufacturers to source raw materials for our products. Any shortages of raw materials would affect our ability to timely deliver our products and could significantly harm our business, and results of operations. Other events that could also cause disruptions to our supply chain include the imposition of additional duties, tariffs and other charges or quotas on imports and exports, natural disasters, severe weather, political instability, war, such as the Russia-Ukraine conflict or the Israel-Hamas war, terrorist attacks, and social unrest and economic instability in the regions in which our raw material suppliers are located, or through which regions they travel.
| 20 |
We currently lack manufacturing capabilities to produce our therapeutic product candidates at commercial-scale quantities and do not have an alternate manufacturing supply, which could negatively impact our ability to meet any future demand for the product.
We expect that we would need to significantly expand our manufacturing capabilities to meet potential demand for our therapeutic product candidates, if approved. Such expansion would require additional regulatory approvals. Even if we increase our manufacturing capabilities, it is possible that we may still lack sufficient capacity to meet demand.
We do not currently have any alternate supply for our products. If the facilities where our products are currently being manufactured or equipment were significantly damaged or destroyed, or if there were other disruptions, delays or difficulties affecting manufacturing capacity or availability of drug supply, including, but not limited to, if such facilities are deemed not in compliance with current Good Manufacturing Practice, or GMP, requirements, future clinical studies and commercial production for our products would likely be significantly disrupted and delayed. It would be both time-consuming and expensive to replace this capacity with third parties, particularly since any new facility would need to comply with the regulatory requirements.
Ultimately, if we are unable to supply our products to meet commercial demand, whether because of processing constraints or other disruptions, delays or difficulties that we experience, our production costs could dramatically increase and sales of our products and their long-term commercial prospects could be significantly damaged.
To be successful, our proposed products must be accepted by the healthcare community, which can be very slow to adopt or unreceptive to new technologies and products.
Our proposed products and those developed by our collaborative partners, if approved for marketing, may not achieve market acceptance since hospitals, physicians, patients or the medical community in general may decide not to accept and use these products. The products that we are attempting to develop represent substantial departures from established treatment methods and will compete with a number of more conventional therapies manufactured and marketed by major pharmaceutical companies. The degree of market acceptance of any of our developed products will depend on a number of factors, including:
| ● | our establishment and demonstration to the medical community of the clinical efficacy and safety of our proposed products; | |
| ● | our ability to create products that are superior to alternatives currently on the market; | |
| ● | our ability to establish in the medical community the potential advantage of our treatments over alternative treatment methods; and | |
| ● | reimbursement policies of government and third-party payers. |
If the healthcare community does not accept our products for any of these reasons, or for any other reason, our business would be materially harmed.
Our business is based on novel technologies that are inherently expensive and risky and may not be understood by or accepted in the marketplace, which could adversely affect our future value.
The clinical development, commercialization and marketing of immuno-oncology therapies are at an early-stage, substantially research-oriented, and financially speculative. To date, very few companies have been successful in their efforts to develop and commercialize an immuno-oncology therapeutic product. In general, such products may be susceptible to various risks, including undesirable and unintended side effects, unintended immune system responses, inadequate therapeutic efficacy, or other characteristics that may prevent or limit their approval or commercial use. Furthermore, the number of people who may use such therapies is difficult to forecast with accuracy. Our future success is dependent on the establishment of a significant market for such therapies and our ability to capture a share of this market with our product candidates.
Our development efforts with our therapeutic product candidates are susceptible to the same risks of failure inherent in the development and commercialization of therapeutic products based on new technologies. The novel nature of immuno-oncology therapeutics creates significant challenges in the areas of product development and optimization, manufacturing, government regulation, third-party reimbursement and market acceptance. For example, the FDA has relatively limited experience regulating such therapies, and there are few approved treatments using such therapy.
| 21 |
Our competition includes fully integrated biotechnology and pharmaceutical companies that have significant advantages over us.
The market for therapeutic immuno-oncology products is highly competitive. We expect that our most significant competitors will be fully integrated and more established pharmaceutical and biotechnology companies or institutions, including major multinational pharmaceutical companies, biotechnology companies and universities and other research institutions. These companies are developing similar products, and they have significantly greater capital resources and research and development, manufacturing, testing, regulatory compliance, and marketing capabilities. Many of these potential competitors may be further along in the process of product development and also operate large, company-funded research and development programs. As a result, our competitors may develop more competitive or affordable products, or achieve earlier patent protection or product commercialization than we are able to achieve. Competitive products may render any products or product candidates that we develop obsolete.
Many of our competitors have substantially greater financial, technical and other resources than we do, such as larger research and development staff and experienced marketing and manufacturing organizations. Additional mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated in certain of our competitors. As a result, these companies may be able to obtain regulatory approval more rapidly than we can and may be more effective in selling and marketing their products. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies. Competition may increase further as a result of advances in the commercial applicability of technologies and greater availability of capital for investment in these industries. Our competitors may succeed in developing, acquiring or licensing drug products that are more effective or less costly to produce or purchase on the market than any product candidate we are currently developing or that we may seek to develop in the future. If approved, our product candidates will face competition from commercially available drugs as well as drugs that are in the development pipelines of our competitors.
Established pharmaceutical companies may invest heavily to accelerate discovery and development of or in-license novel compounds that could make our product candidates less competitive. In addition, any new product that competes with an approved product must demonstrate compelling advantages in efficacy, convenience, tolerability and safety in order to overcome price competition and to be commercially successful. Accordingly, our competitors may succeed in obtaining patent protection, receiving FDA, EMA or other regulatory approval, or discovering, developing and commercializing medicines before we do, which would have a material adverse impact on our business and ability to achieve profitability from future sales of our approved product candidates, if any.
If competitors develop and market products that are more effective, safer or less expensive than our product candidates or offer other advantages, our commercial prospects will be limited.
Our therapeutic immuno-oncology (IO) development programs face, and will continue to face, intense competition from pharmaceutical, biopharmaceutical and biotechnology companies, as well as numerous academic and research institutions and governmental agencies engaged in drug discovery activities or funding, both in the United States and abroad. Some of these competitors are pursuing the development of drugs and other therapies that target the same diseases and conditions that we are targeting with our product candidates. According to Global Data, Thematic Research: Immuno-Oncology (March 2021), as of December 2020, there are 4,822 industry-sponsored clinical trials for immuno-oncology with 422 drugs in development. Phase 2 trials constitute the majority of the IO pipeline, followed by early-stage molecules in Phase 1/2 and Phase 1. For late-stage pipeline products, 484 clinical trials are ongoing in Phase 3, and 51 are in Phase 2/3 development. There are currently 22 marketed immuno-oncology agents. Cancer vaccine products lead the category with 9 products followed by checkpoint modulators with 8 approved drugs. The indications with the most marketed IO agents in the United States are metastatic melanoma and non-small cell lung cancer, with 6 approved products each. The market value of bispecific antibodies, cancer vaccines, checkpoint modulators, cell therapies, and oncolytic viruses globally has increased sharply in the past 10 years with nearly $29 billion in 2019 compared to $370 million in 2010 .
As a general matter, we also face competition from many companies that are researching and developing cell therapies. Many of these companies have financial and other resources substantially greater than ours. In addition, many of these competitors have significantly greater experience in testing pharmaceutical and other therapeutic products, obtaining FDA and other regulatory approvals, and marketing and selling. If we obtain regulatory approval for any of our product candidates, we also will be competing with respect to manufacturing efficiency and marketing capabilities, areas in which we have limited or no commercial-scale experience. Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources’ being concentrated by our competitors. Competition may increase further as a result of advances made in the commercial applicability of our technologies and greater availability of capital for investment in these fields.
If we are unable to keep up with rapid technological changes in our field or compete effectively, we will be unable to operate profitably.
We are engaged in activities in the biotechnology field, which is characterized by extensive research efforts and rapid technological progress. If we fail to anticipate or respond adequately to technological developments, our ability to operate profitably could suffer. Research and discoveries by other biotechnology, pharmaceutical or other companies may render our technologies or potential products or services uneconomical or result in products superior to those we develop. Similarly, any technologies, products or services we develop may not be preferred to any existing or newly developed technologies, products or services.
| 22 |
We may not be able to obtain third-party patient reimbursement or favorable product pricing, which would reduce our ability to operate profitably.
Our ability to successfully commercialize certain of our proposed products in the human therapeutic field may depend to a significant degree on patient reimbursement of the costs of such products and related treatments at acceptable levels from government authorities, private health insurers and other organizations, such as health maintenance organizations. Reimbursement in the United States or foreign countries may not be available for any products we may develop, and, if available, may be decreased in the future. Also, reimbursement amounts may reduce the demand for, or the price of, our products with a consequent harm to our business. We cannot predict what additional regulation or legislation relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future or what effect such regulation or legislation may have on our business. If additional regulations are overly onerous or expensive, or if healthcare-related legislation makes our business more expensive or burdensome than originally anticipated, we may be forced to significantly downsize our business plans or completely abandon our business model.
We may be subject to litigation that will be costly to defend or pursue and uncertain in its outcome.
Our business may bring us into conflict with our licensees, licensors or others with whom we have contractual or other business relationships, or with our competitors or others whose interests differ from ours. If we are unable to resolve those conflicts on terms that are satisfactory to all parties, we may become involved in litigation brought by or against us. That litigation is likely to be expensive and may require a significant amount of management’s time and attention, at the expense of other aspects of our business. The outcome of litigation is always uncertain, and in some cases could include judgments against us that require us to pay damages, enjoin us from certain activities, or otherwise affect our legal or contractual rights, which could have a significant adverse effect on our business.
We are exposed to the risk of liability claims, for which we may not have adequate insurance.
Since we participate in the pharmaceutical industry, we may be subject to liability claims by employees, customers, end users and third parties. We intend to obtain proper insurance, however, there can be no assurance that any liability insurance we purchase will be adequate to cover claims asserted against us or that we will be able to maintain such insurance in the future. We intend to adopt prudent risk-management programs to reduce these risks and potential liabilities, however, we have not taken any steps to create these programs and have no estimate as to the cost or time required to do so and there can be no assurance that such programs, if and when adopted, will fully protect us. We may not be able to put risk management programs in place, or obtain insurance, if we are unable to retain the necessary expertise and/or are unsuccessful in raising necessary capital in the future. Our failure to obtain appropriate insurance, or to adopt and implement effective risk-management programs, as well as any adverse rulings in any legal matters, proceedings and other matters could have a material adverse effect on our business.
Preclinical and clinical trials are conducted during the development of potential products and other treatments to determine their safety and efficacy for use by humans. Notwithstanding these efforts, when our treatments are introduced into the marketplace, unanticipated side effects may become evident. Manufacturing, marketing, selling and testing our product candidates under development or to be acquired or licensed, entails a risk of product liability claims. We could be subject to product liability claims if our product candidates, processes, or products under development fail to perform as intended. Even unsuccessful claims could result in the expenditure of funds in litigation and the diversion of management time and resources, and could damage our reputation and impair the marketability of our product candidates and processes. While we plan to maintain liability insurance for product liability claims, we may not be able to obtain or maintain such insurance at a commercially reasonable cost. If a successful claim were made against us, and we lacked insurance or the amount of insurance were inadequate to cover the costs of defending against or paying such a claim or the damages payable by us, we would experience a material adverse effect on our business, financial condition and results of operations.
We could be subject to product liability lawsuits based on the use of our product candidates in clinical testing or, if obtained, following marketing approval and commercialization. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to cease clinical testing or limit commercialization of our product candidates.
We could be subject to product liability lawsuits if any product candidate we develop allegedly causes injury or is found to be otherwise unsuitable for human use during product testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the product, negligence, strict liability and a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our product candidates, if approved. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| ● | decreased demand for our product candidates; | |
| ● | withdrawal of clinical trial participants; | |
| ● | initiation of investigations by regulators; | |
| ● | costs to defend the related litigation; | |
| ● | a diversion of management’s time and our resources; | |
| ● | substantial monetary awards to trial participants or patients; | |
| ● | product recalls, withdrawals or labeling, marketing or promotional restrictions; | |
| ● | loss of revenues from product sales; and | |
| ● | the inability to commercialize our product candidates. |
| 23 |
Our inability to retain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the clinical testing and commercialization of products we develop. We may wish to obtain additional such insurance covering studies or trials in other countries should we seek to expand those clinical trials or commence new clinical trials in other jurisdictions or increase the number of patients in any clinical trials we may pursue. We also may determine that additional types and amounts of coverage would be desirable at later stages of clinical development of our product candidates or upon commencing commercialization of any product candidate that obtains required approvals. However, we may not be able to obtain any such additional insurance coverage when needed on acceptable terms or at all. If we do not obtain or retain sufficient product liability insurance, we could be responsible for some or all of the financial costs associated with a product liability claim relating to our preclinical and clinical development activities, in the event that any such claim results in a court judgment or settlement in an amount or of a type that is not covered, in whole or in part, by any insurance policies we may have or that is in excess of the limits of our insurance coverage. We may not have, or be able to obtain, sufficient capital to pay any such amounts that may not be covered by our insurance policies.
We rely on third parties to conduct preclinical and clinical trials of our product candidates. If these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may not be able to obtain regulatory approval for or commercialize our product candidates and our business could be substantially harmed.
We rely, and expect to continue to rely, upon third-party clinical research organizations (CROs) to execute our preclinical and clinical trials and to monitor and manage data produced by and relating to those trials. However, we may not be able to establish arrangements with CROs when needed or on terms that are acceptable to us, or at all, which could negatively affect our development efforts with respect to our drug product candidates and materially harm our business, operations and prospects.
We will have only limited control over the activities of the CROs we will engage to conduct our clinical trials. Nevertheless, we are responsible for ensuring that each of our studies is conducted in accordance with the applicable protocol, legal, regulatory and scientific standards, and our reliance on any of the CROs does not relieve us of our regulatory responsibilities. Based on our present expectations, we, our CROs and our clinical trial sites are required to comply with good clinical practices (GCPs), for all our product candidates in clinical development. Regulatory authorities enforce GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we or any of our CROs fail to comply with applicable GCPs, the clinical data generated in the applicable trial may be deemed unreliable and the FDA, EMA or comparable foreign regulatory authorities may require us to perform additional clinical trials before approving a product candidate for marketing, which we may not have sufficient cash or other resources to support and which would delay our ability to generate revenue from any sales of such product candidate. In addition, our clinical trials are required to be conducted with product produced in compliance with current good manufacturing practice requirements, or cGMP. Our CROs’ failure to comply with those regulations may require us to repeat clinical trials, which would also require significant cash expenditures and delay the regulatory approval process.
Agreements governing relationships with CROs generally provide those CROs with certain rights to terminate a clinical trial under specified circumstances. If a CRO that we have engaged terminates its relationship with us during the performance of a clinical trial, we would be forced to seek an engagement with a substitute CRO, which we may not be able to do on a timely basis or on commercially reasonable terms, if at all, and the applicable trial would experience delays or may not be completed. In addition, our CROs are not our employees, and except for remedies available to us under any agreements we enter with them, we are unable to control whether or not they devote sufficient time and resources to our clinical, nonclinical and preclinical programs. If CROs do not successfully carry out their contractual duties or obligations or meet expected deadlines, if they need to be replaced or if the quality or accuracy of the clinical data they obtain is compromised due to a failure to adhere to our clinical protocols, regulatory requirements or for other reasons, our clinical trials may be extended, delayed or terminated and we may not be able to obtain regulatory approval for, or successfully commercialize, the affected product candidates. As a result, our operations and the commercial prospects for the effected product candidates would be harmed, our costs could increase and our ability to generate revenues could be delayed.
| 24 |
We contract with third parties for the supply of product candidates for clinical testing and expect to contract with third parties for the manufacturing of our product candidates for large-scale testing and commercial supply. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We anticipate continuing our engagement of third parties to provide our clinical supply as we advance our product candidates into and through clinical development, and we depend on third parties to produce and maintain sufficient quantities of material to supply our clinical trials. If these third parties do not produce and maintain adequate supplies of clinical material, our development efforts could be significantly delayed, or could incur substantially higher costs. We expect in the future to use third parties for the manufacture of our product candidates for clinical testing, as well as for commercial manufacture. We plan to enter into long-term supply agreements with several manufacturers for commercial supplies. We may be unable to reach agreement on satisfactory terms with contract manufacturers to manufacture our product candidates. Additionally, the facilities to manufacture our product candidates must be the subject of a satisfactory inspection before the FDA or other regulatory authorities approve a marketing authorization for the product candidate manufactured at that facility. We will depend on these third-party manufacturers for compliance with the FDA’s and international regulatory authority requirements for the manufacture of our finished products. We do not control the manufacturing process of, and are completely dependent on, our contract manufacturers for compliance with cGMP. If our manufacturers cannot successfully manufacture material that conforms to our specifications and the FDA and other regulatory authorities’ cGMP requirements, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved, and may subject us to recalls or enforcement action for products already on the market.
If any of our product candidates are approved and contract manufacturers fail to deliver the required commercial quantities of finished product on a timely basis and at commercially reasonable prices, and we are unable to find one or more replacement manufacturers capable of production at a substantially equivalent cost, in substantially equivalent volumes and quality and on a timely basis, we would likely be unable to meet demand for our products and could lose potential revenue. It may take several years to establish an alternative source of supply for our product candidates and to have any such new source approved by the FDA or any other relevant regulatory authorities.
We currently have no marketing and sales force. If we are unable to establish effective marketing and sales capabilities or enter into agreements with third parties to market and sell our product candidates, we may not be able to effectively market and sell our product candidates, if approved, or generate product revenues.
We currently do not have a marketing or sales team for the marketing, sales and distribution of any of our product candidates that are able to obtain regulatory approval. To commercialize any product candidates, we must build on a territory-by-territory basis marketing, sales, distribution, managerial and other non-technical capabilities or make arrangements with third parties to perform these services, and we may not be successful in doing so. If our product candidates receive regulatory approval, we intend to establish an internal sales and marketing team with technical expertise and supporting distribution capabilities to commercialize our product candidates, which will be expensive and time consuming and will require significant attention of our executive officers to manage. Any failure or delay in the development of our internal sales, marketing and distribution capabilities would adversely impact the commercialization of any of our products that we obtain approval to market. With respect to the commercialization of all or certain of our product candidates, we may choose to collaborate, either globally or on a territory-by-territory basis, with third parties that have direct sales forces and established distribution systems, either to augment our own sales force and distribution systems or in lieu of our own sales force and distribution systems. If we are unable to enter into such arrangements when needed on acceptable terms or at all, we may not be able to successfully commercialize any of our product candidates that receive regulatory approval or any such commercialization may experience delays or limitations. If we are not successful in commercializing our product candidates, either on our own or through collaborations with one or more third parties, our future product revenue will suffer and we may incur significant additional losses.
Our business and operations would suffer in the event of system failures.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we have not experienced any such system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from completed or ongoing or planned clinical trials could result in delays in our regulatory approval efforts and we may incur substantial costs to attempt to recover or reproduce the data. If any disruption or security breach resulted in a loss of or damage to our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and/or the further development of our product candidates could be delayed.
| 25 |
We face risks associated with security breaches or cyber-attacks.
We face risks associated with security breaches or cyber-attacks of our computer systems or those of our third-party representatives, vendors, and service providers. Armed conflicts in the Middle East and between Russia and Ukraine, and tensions with countries such as Iran and North Korea and resulting geopolitical uncertainties also could result in an increase in cyberattacks that could either directly or indirectly impact our operations. Although we have implemented security procedures and controls to address these threats, such as firewalls, encryption, access controls, and employee training programs, cybersecurity threats are dynamic and evolving and our systems may still be vulnerable to theft, loss or misuse of data, including proprietary or confidential information, relating to our business, products, employees, suppliers and customers; disruption due to computer viruses and programming errors; attacks by third parties including destruction of data or demanding ransom to return control of our systems and services; or similar disruptive problems.
Our operations are vulnerable to interruption by natural disasters, power loss, terrorist activity and other events beyond our control, the occurrence of which could materially harm our business.
Businesses located in California have, in the past, been subject to electrical blackouts as a result of a shortage of available electrical power, and any future blackouts could disrupt our operations. We are vulnerable to a major earthquake, wildfire and other natural disasters, and we have not undertaken a systematic analysis of the potential consequences to our business as a result of any such natural disaster and do not have an applicable recovery plan in place. We do not carry any business interruption insurance that would compensate us for actual losses from interruption of our business that may occur, and any losses or damages incurred by us could cause our business to materially suffer.
Epidemic or pandemic outbreaks such as COVID-19 (coronavirus), natural disasters, whether or not caused by climate change, unusual weather conditions, terrorist acts and political events, could disrupt business and result in halting our clinical trials and otherwise adversely affect our financial performance.
The occurrence of one or more natural disasters, such as tornadoes, hurricanes, fires, floods and earthquakes, unusual weather conditions, epidemic outbreaks, terrorist attacks or disruptive political events in certain regions where our operations are located could adversely affect our business. Epidemic or pandemic outbreaks, such as COVID-19 (coronavirus) could impact our management and our ability to conduct clinical trials. This also may affect the market conditions that would limit our ability to raise additional capital. This could have a sustained material adverse effect on our business, financial condition and results of operations.
Risks Related to Our Common Stock
There has been a limited public market for our common stock , and we do not know whether one will develop to provide adequate liquidity. Furthermore, the trading price for our common stock, should an active trading market develop, may be volatile and could be subject to wide fluctuations in per-share price.
Our common stock is now listed on the Nasdaq Capital Market under the trading symbol “GTBP”; historically, however, there has been a limited public market for our common stock. We cannot assure you that an active trading market for our common stock will develop or be sustained. The liquidity of any market for the shares of our common stock will depend on a number of factors, including:
| ● | the number of stockholders; | |
| ● | our operating performance and financial condition; | |
| ● | the market for similar securities; | |
| ● | the extent of coverage of us by securities or industry analysts; and | |
| ● | the interest of securities dealers in making a market in the shares of our common stock. |
Even if an active trading market develops, the market price for our common stock may be highly volatile and could be subject to wide fluctuations. In addition, the price of shares of our common stock could decline significantly if our future operating results fail to meet or exceed the expectations of market analysts and investors and actual or anticipated variations in our quarterly operating results could negatively affect our share price.
The volatility of the price of our common stock may also be impacted by the risks discussed under this “Risk Factors” section, in addition to other factors, including:
| ● | developments in the financial markets and worldwide or regional economies; | |
| ● | announcements of innovations or new products or services by us or our competitors; | |
| ● | announcements by the government relating to regulations that govern our industry; | |
| ● | significant sales of our common stock or other securities in the open market; | |
| ● | variations in interest rates; | |
| ● | changes in the market valuations of other comparable companies; and | |
| ● | changes in accounting principles. |
| 26 |
Our outstanding warrants and options may affect the market price of our common stock
As of December 31, 2024, we had 2,234,328 shares of common stock outstanding and issued and had outstanding warrants for the purchase of up to 1,120,429 additional shares of common stock at a weighted average exercise price of $18.85 per share, all of which are exercisable (subject to certain beneficial ownership limitations). In addition, we had outstanding options for the purchase of up to 124,600 additional shares of common stock at a weighted average exercise price of $32.69 per share, 105,802 of which are exercisable. The amount of common stock reserved for issuance may have an adverse impact on our ability to raise capital and may affect the price and liquidity of our common stock in the public market. In addition, the issuance of these shares of common stock will have a dilutive effect on current stockholders’ ownership.
Because our common stock may be deemed a low-priced “penny” stock, an investment in our common stock should be considered high-risk and subject to marketability restrictions.
Historically, the trading price of our common stock has been $5.00 per share or lower, and deemed a penny stock, as defined in Rule 3a51-1 under the Exchange Act, and subject to the penny stock rules of the Exchange Act specified in rules 15g-1 through 15g-100. Those rules require broker–dealers, before effecting transactions in any penny stock, to:
| ● | deliver to the customer, and obtain a written receipt for, a disclosure document; |
| ● | disclose certain price information about the stock; |
| ● | disclose the amount of compensation received by the broker-dealer or any associated person of the broker-dealer; |
| ● | send monthly statements to customers with market and price information about the penny stock; and |
| ● | in some circumstances, approve the purchaser’s account under certain standards and deliver written statements to the customer with information specified in the rules. |
Consequently, the penny stock rules may restrict the ability or willingness of broker-dealers to sell the common stock and may affect the ability of holders to sell their common stock in the secondary market and the price at which such holders can sell any such securities. These additional procedures could also limit our ability to raise additional capital in the future.
Financial Industry Regulatory Authority (“FINRA”) sales practice requirements may also limit a stockholder’s ability to buy and sell our common stock, which could depress the price of our common stock.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require a broker-dealer to have reasonable grounds for believing that the investment is suitable for that customer before recommending an investment to a customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. Thus, the FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our shares of common stock, have an adverse effect on the market for our shares of common stock, and thereby depress our price per share of common stock.
If securities or industry analysts do not publish research or reports about our business, or if they issue an adverse or misleading opinion regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock may be influenced by the research and reports that industry or securities analysts publish about us or our business. We currently have research coverage by only one securities analyst, and we may never obtain research coverage by additional analysts. If no or few securities or industry analysts commence coverage of us, the trading price for our common stock may be negatively affected. In the event that we receive additional securities or industry analyst coverage, if any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
| 27 |
Anti-takeover provisions may limit the ability of another party to acquire us, which could cause our stock price to decline.
Delaware law and our restated certificate of incorporation (“certificate of incorporation”), our restated bylaws (“bylaws”) and other governing documents contain provisions that could discourage, delay or prevent a third party from acquiring us, even if doing so may be beneficial to our stockholders, which could cause our stock price to decline. In addition, these provisions could limit the price investors would be willing to pay in the future for shares of our common stock.
We do not currently or for the foreseeable future intend to pay dividends on our common stock.
We have never declared or paid any cash dividends on our common stock. We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. As a result, any return on your investment in our common stock will be limited to the appreciation in the price of our common stock, if any.
Our management will have broad discretion as to the use of the proceeds from securities offerings, and may not use the proceeds effectively.
Our management will have broad discretion as to the application of the net proceeds from securities we offer for sale. Currently, we intend to use the net proceeds from any such sale for working capital and general corporate purposes, including the further development of our product candidates that are currently undergoing clinical trials, our product candidates that we expect to submit an investigational new drug application for in the near term, and our product candidates that are pre-clinical. See “Use of Proceeds.” Purchasers will not have the opportunity, as part of their investment decision, to assess whether these proceeds are being used appropriately. Our management may use the net proceeds for corporate purposes that may not improve our financial condition or market value, which could cause the price of our securities to decline.
We will need additional capital to conduct our operations and develop our products, and our ability to obtain the necessary funding is uncertain.
We have used a significant amount of cash since inception to finance the continued development and testing of our product candidates, and we expect to need substantial additional capital resources to develop our product candidates going forward and launch and commercialize any product candidates for which we receive regulatory approval.
We may not be successful in generating and/or maintaining operating cash flow, and the timing of our capital expenditures and other expenditures may not result in cash sufficient to sustain our operations through the commercialization of our product candidates. If financing is not sufficient and additional financing is not available or available only on terms that are detrimental to our long-term survival, it could have a material adverse effect on our ability to continue to function. The timing and degree of any future capital requirements will depend on many factors, including:
| ● | accuracy of the assumptions underlying our estimates for capital needs in 2025 and beyond; |
| ● | scientific and clinical progress in our research and development programs; |
| ● | the magnitude and scope of our research and development programs and our ability to establish, enforce and maintain strategic arrangements for research, development, clinical testing, manufacturing and marketing; |
| ● | our progress with pre-clinical development and clinical trials; |
| ● | the time and costs involved in obtaining regulatory approvals; |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; and |
| ● | the number and type of product candidates that we pursue. |
Additional financing through strategic collaborations, public or private equity or debt financings or other financing sources may not be available on acceptable terms, or at all. Additional equity financing could result in significant dilution to our stockholders, and any debt financings will likely involve covenants restricting our business activities. Further, if we obtain additional funds through arrangements with collaborative partners, these arrangements may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise seek to develop and commercialize on our own.
If sufficient capital is not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or product development initiatives, any of which could have a material adverse effect on our financial condition or business prospects.
| 28 |
Our common stock may be at risk for delisting from the Nasdaq Capital Market in the future if we do not maintain compliance with Nasdaq’s continued listing requirements. Delisting could adversely affect the liquidity of our common stock and the market price of our common stock could decrease.
Our common stock is currently listed on Nasdaq. Nasdaq has minimum requirements that a company must meet in order to remain listed on Nasdaq, including corporate governance standards and a requirement that we maintain a stockholders’ equity above $2,500,000 as set forth in Nasdaq Listing Rule 5550(b)(1).
On November 21, 2024, the Company received a letter from Nasdaq notifying the Company that its amount of stockholders’ equity has fallen below the $2,500,000 required minimum for continued listing set forth in Nasdaq Listing Rule 5550(b)(1).
Nasdaq’s Letter has no immediate effect on the listing of the Company’s common stock, and its common stock will continue to trade on The Nasdaq Capital Market under the symbol “GTBP” at this time. Pursuant to Nasdaq Listing Rules, the Company provided Nasdaq with a plan to achieve and sustain compliance on December 31, 2024. If Nasdaq accepts the Company’s plan to regain compliance, Nasdaq may grant an extension of up to 180 calendar days from the date of the Letter to evidence compliance. If Nasdaq does not accept the Company’s plan to regain compliance, the Company will have the opportunity to appeal the decision to a Nasdaq Hearings Panel. The Company intends to submit to Nasdaq, within the requisite time period, a plan to regain compliance with Listing Rule 5550(b)(1). There can be no assurance that Nasdaq will accept the Company’s plan, that the Company will be able to regain compliance with Listing Rule 5550(b)(1) or that the Company will be able meet the continued listing requirements during any compliance period that may be granted by Nasdaq.
In the future, if we fail to maintain such minimum requirements and a final determination is made by Nasdaq that our common stock must be delisted, the liquidity of our common stock would be adversely affected and the market price of our common stock could decrease. In addition, if delisted, we would no longer be subject to Nasdaq rules, including rules requiring us to have a certain number of independent directors and to meet other corporate governance standards. Our failure to be listed on Nasdaq or another established securities market would have a material adverse effect on the value of your investment in us.
Failure to achieve and maintain effective internal controls in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 could result in a restatement of our financial statements, cause investors to lose confidence in our financial statements and our company and have a material adverse effect on our business and stock price.
We produce our financial statements in accordance with accounting principles generally accepted in the United States, or GAAP. Effective internal controls are necessary for us to provide reliable financial reports to help mitigate the risk of fraud and to operate successfully as a publicly traded company. As a public company, we are required to document and test our internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or Section 404. Further, Section 404 requires annual management assessments of the effectiveness of our internal controls over financial reporting.
Testing and maintaining internal controls can divert our management’s attention from other matters that are important to our business. We may not be able to conclude on an ongoing basis that we have effective internal controls over financial reporting in accordance with Section 404. If we are unable to conclude that we have effective internal controls over financial reporting, investors could lose confidence in our reported financial information and our company, which could result in a decline in the market price of our common stock, and cause us to fail to meet our reporting obligations in the future, which in turn could impact our ability to raise additional financing if needed in the future.
ITEM 1B. UNRESOLVED STAFF COMMENTS
Not applicable.
| 29 |
ITEM 1C. CYBERSECURITY
| ● | Governance
and Oversight: | |
| ● | Risk
Assessment and Management: | |
| ● | Information Security Policies and Procedures: We have established information security policies and procedures that govern the use, protection, and handling of sensitive information. These policies cover areas such as data encryption, access controls, password management, incident response, and employee training. | |
| ● | Network and Infrastructure Security: We employ a range of technical measures to secure our network and infrastructure, including firewalls, intrusion detection and prevention systems, and vulnerability assessments. We also use encryption to protect data both in transit and at rest. | |
| ● | Employee Training and Awareness: We provide cybersecurity training to employees to raise awareness of the latest threats and best practices for protecting sensitive information. This includes training on how to recognize phishing attempts, handling secure data, and reporting security incidents. | |
| ● | Third-Party
Risk Management: | |
| ● | Compliance and Reporting: We comply with all applicable laws and regulations related to cybersecurity, including data protection and privacy laws. |
ITEM 2. PROPERTIES
Effective as of July 1, 2024, the Company became a fully remote company. We operate in a virtual environment and do not have a physical office space or headquarters.
ITEM 3. LEGAL PROCEEDINGS
Ohri Matter
On July 22, 2024, the Company filed an AAA Arbitration Demand against Manu Ohri, its former Chief Financial Officer. In the Demand, the Company asserts claims against Mr. Ohri for breach of his fiduciary duties and breach of contract and seeks a declaratory judgment providing that the Company may characterize Mr. Ohri’s termination as “for cause” under his Employment Agreement, and that the Company may revoke the Separation Agreement entered into between the Company and Mr. Ohri prior to the Company learning of Mr. Ohri’s breaches. In addition to the declaratory judgment, the Company seeks damages arising from Mr. Ohri’s violations, and attorneys’ fees and any forum and arbitration fees. On September 3, 2024, Mr. Ohri filed both a general denial of the Company’s claims against him and counterclaims for breach of his Employment Agreement and Separation Agreement. A final hearing is scheduled for June 10th through June 12th, 2025 in Los Angeles, California. At this stage in the proceedings the Company is not able to determine the probability of the outcome of this matter or a range of reasonably expected losses, if any. The Company believes it has recorded an appropriate accrual for the matter.
Berk Matter
On November 14, 2023, former interim Chief Executive Officer, Dr. Gregory Berk filed a lawsuit in the U.S. District Court for the District of Massachusetts alleging that the Company discriminated and retaliated against Dr. Berk for engaging in protected whistleblowing activity in violation of the Sarbanes Oxley Act (“SOX”). Although the Company vigorously defended this matter and believes it to be without merit, the lawsuit was dismissed with prejudice on December 4, 2024 as a result of the parties’ settlement. The Company has recorded the monetary settlement for this matter in its financial statements.
| 30 |
TWF Global Matter
On May 24, 2023, TWF Global, LLC (“TWF”) filed a Complaint in the California Superior Court for the County of Los Angeles naming the Company as defendant. The Complaint alleges that TWF is the holder of two Convertible Promissory Notes (“Notes”) and that the Company did not deliver shares of common stock due on conversion in February 2021. TWF was seeking per diem liquidated damages based on the terms of alleged Notes. On July 14, 2023, the Company filed a motion to dismiss for improper forum because the terms of the Notes, as alleged, require disputes to be filed in New York state and federal courts. TWF voluntarily dismissed its Complaint before the California Superior Court of Los Angeles without prejudice. The Company subsequently filed a Summons and Complaint for Interpleader against TWF and Z-One LLC before the Supreme Court of the State of New York County of New York, asking the Supreme Court to determine if the Company’s shares of common stock are properly registered to TWF or Z-One LLC, as both of these entities have made conflicting demands for registration of the shares of common stock. On February 5, 2024, the Company filed a motion for entry of default against TWF, seeking an order directing the Company to register the shares of common stock in the name of Z-One and that the Company be released from all associated liability and claims. The Court denied the motion without prejudice and agreed to reconsider the motion without further briefing upon the filing of a supplemental party affidavit. On May 9, 2024, Z-One filed a motion for summary judgement seeking dismissal of the action, representing that Z-One and TWF have settled their dispute over the entitlement to the Company’s shares of common stock and there is no remaining dispute before the Court. On May 21, 2024, the Company filed a supplemental affidavit in support of its motion for entry of default. On November 14, 2024, the Court held a hearing on the parties’ motions, at which the Court found that the motion for entry of default was mooted by the settlement agreement between Z-One and TWF. The Court ordered that the case be dismissed. On February 17, 2025, Z-One, LLC filed a Summons with Notice in the Supreme Court of the State of New York, County of New York. Z-One alleges that it was assigned TWF’s rights under the Notes and seeks enforcement and damages. The Company intends to demand a complaint, and seek dismissal of the case. The Company believes that the claims related to the Notes are without merit, and will continue to vigorously defend against these claims.
Handelman Matter
On May 13, 2022, the Company made an arbitration demand upon Michael Handelman, its former Chief Financial Officer, asserting that he breached his fiduciary duty by misappropriating Company funds and shares of common stock, among other things. The Company sought among other relief, monetary damages estimated at $470,000; the return of 13,902 shares of our common stock received without authorization; and an award of the Company’s attorneys’ fees and any forum and arbitration fees. In May 2024, the Arbitrator issued the final award in favor of the Company which awarded the Company its legal fees in the amount of $473,000 plus costs in the amount of $19,000 and the return of the disputed 13,902 shares of common stock, which were returned to the Company in June 2024. As of December 31, 2024, the Company has not recorded any amounts with regards to the monetary award of $473,000, due to the uncertainty of its collectability.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
| 31 |
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Our common stock is traded on the Nasdaq Capital Market under the trading symbol “GTBP.” Until May 2009, our common stock was traded on the OTC Bulletin Board (“OTCBB”) under the symbol “OXIS.” From May 20, 2009 until March 11, 2010, our common stock was traded on Pink OTC Markets Inc. trading platform under the symbol “OXIS.” From January 2015 to August 2017, our common stock was quoted on the OTCQB under the “OXIS” trading symbol. From August 2017 to February 11, 2021, our common stock was quoted on the OTCQB under the “GTBP” trading symbol.
Stockholders
As of February 24, 2025, there were 28 stockholders of record, which does not include stockholders who hold their shares in “street name.” The transfer agent for our common stock is ComputerShare Limited, whose address is 8742 Lucent Blvd., Suite 225, Highland Ranch, CO 80129.
Dividends
We have not paid any dividends on our common stock to date and do not anticipate that we will pay dividends in the foreseeable future. Any payment of cash dividends on our common stock in the future will be dependent upon the amount of funds legally available, our earnings, if any, our financial condition, our anticipated capital requirements and other factors that the Board of Directors may think are relevant. However, we currently intend for the foreseeable future to follow a policy of retaining all of our earnings, if any, to finance the development and expansion of our business and, therefore, do not expect to pay any dividends on our common stock in the foreseeable future.
Equity Compensation Plan Information
Information relating to compensation plans under which our equity securities are authorized for issuance is set forth in Item 12 of this report under “Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.”
Recent Issuances of Unregistered Securities
The Company made the following issuances of its unregistered securities pursuant exemptions contained in Section 4(a)(2) or 3(a)(9) of the Securities Act and/or Rule 506 of Regulation D promulgated thereunder during the year ended December 31, 2024:
| ● | On April 30, 2024, the Company issued 36,018 shares of common stock to settle $278,500 of vendor accounts payable. The shares were valued at the month-end closing price of the Company’s common stock for the months for which services were provided by the vendor. |
| ● | On May 23, 2024, the Company issued 740,000 common warrants, each to purchase one share of common stock at an exercise price equal to $4.35 and are exercisable immediately upon issuance and will expire on the date that is five years following the date of issuance. |
| ● | On May 23, 2024, the Company issued placement agent warrants to the placement agent to purchase up to 88,800 shares of common stock as part of the compensation payable to the placement agent in connection with such offering at an exercise price of $5.4375 per share and will expire five years from the commencement of sales of the offering. |
| ● | On June 30, 2024, the Company issued 91,579 shares of common stock to settle $531,300 of vendor accounts payable. The shares were valued at the month-end closing price of the Company’s common stock for the months for which services were provided by the vendor. |
Issuer Purchases of Equity Securities
We did not repurchase any shares during the fourth quarter of the fiscal year covered by this Annual Report on Form 10-K.
ITEM 6. [RESERVED]
| 32 |
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Some of the statements in this Annual Report on Form 10-K are “forward-looking statements” within the meaning of the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding our current beliefs, goals and expectations about matters such as our expected financial position and operating results, our business strategy and our financing plans. The forward-looking statements in this report are not based on historical facts, but rather reflect the current expectations of our management concerning future results and events. The forward-looking statements generally can be identified by the use of terms such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “foresee,” “may,” “guidance,” “estimate,” “potential,” “outlook,” “target,” “forecast,” “likely” or other similar words or phrases. Similarly, statements that describe our objectives, plans or goals are, or may be, forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be different from any future results, performance and achievements expressed or implied by these statements. We cannot guarantee that our forward-looking statements will turn out to be correct or that our beliefs and goals will not change. Our actual results could be very different from and worse than our expectations for various reasons. You should carefully review all information, including the discussion of risk factors under “Part I. Item 1A: Risk Factors” and elsewhere in this annual report. Any forward-looking statements in the Form 10-K are made only as of the date hereof and, except as may be required by law, we do not have any obligation to publicly update any forward-looking statements contained in this Form 10-K to reflect subsequent events or circumstances.
Overview
We are a clinical stage biopharmaceutical company focused on the development and commercialization of novel immuno-oncology products based off our proprietary Tri-specific Killer Engager (TriKE®) technology platform. Our TriKE® platform generates proprietary therapeutics designed to harness and enhance the cancer killing abilities of a patient’s own natural killer cells, or NK cells. Once bound to an NK cell, our moieties are designed to enhance the NK cell, and precisely direct it to one or more specifically-targeted proteins expressed on a specific type of cancer cell or virus infected cell, ultimately resulting in the targeted cell’s death. TriKE® is composed of recombinant fusion proteins and interleukin 15 (IL-15), can be designed to target any number of tumor antigens on hematologic malignancies, sarcomas or solid tumors and do not require patient-specific customization.
Critical Accounting Policies
The preparation of our financial statements in conformity with accounting principles generally accepted in the United States, or GAAP, requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosure of contingent assets and liabilities. When making these estimates and assumptions, we consider our historical experience, our knowledge of economic and market factors and various other factors that we believe to be reasonable under the circumstances. Actual results may differ under different estimates and assumptions. The accounting estimates and assumptions discussed in this section are those that we consider to be the most critical to gain an understanding of our financial statements because they inherently involve significant judgments and uncertainties.
Use of Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Significant estimates include accruals for potential liabilities, assumptions used in deriving the fair value of warrant liabilities, valuation of equity instruments issued for services, and valuation of deferred tax assets. Actual results could differ from those estimates.
Warrant Liability
We evaluate our financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging”. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations.
Our use of derivative financial instruments is generally limited to warrants issued by us that do not meet the criteria for equity treatment and are recorded as liabilities. We do not use financial instruments or derivatives for any trading purposes.
Stock-Based Compensation
We periodically issue stock-based compensation to officers, directors, employees, and consultants for services rendered. Such issuances vest and expire according to terms established at the issuance date.
Stock-based payments made to officers, directors, employees, and consultants in exchange for goods and services, including grants of employee stock options, are recognized in the financial statements based on their grant date fair values in accordance with ASC 718, Compensation-Stock Compensation. Stock based payments to officers, directors, employees, and consultants, which are generally time vested, are measured at the grant date fair value and depending on the conditions associated with the vesting of the award, compensation cost is recognized on a straight-line or graded basis over the vesting period. Recognition of compensation expense for non-employees is in the same period and manner as if we had paid cash for the services. The fair value of stock options granted is estimated using the Black-Scholes option-pricing model, which uses certain assumptions related to risk-free interest rates, expected volatility, expected life, and future dividends. The assumptions used in the Black-Scholes option pricing model could materially affect compensation expense recorded in future periods.
| 33 |
Results of Operations
Comparison of the Years Ended December 31, 2024 and 2023
Operating Expenses
| Years Ended December 31, | ||||||||||||||||
| 2024 | 2023 | $ Change | % Change | |||||||||||||
| Operating Expenses: | ||||||||||||||||
| Research and development | $ | 5,798,000 | $ | 6,466,000 | $ | (668,000 | ) | (10 | )% | |||||||
| Selling, general and administrative | 8,336,000 | 4,890,000 | 3,446,000 | 70 | % | |||||||||||
| Stock compensation | 230,000 | 2,220,000 | (1,990,000 | ) | (90 | )% | ||||||||||
| Total Operating Expenses | $ | 14,364,000 | $ | 13,576,000 | $ | 788,000 | 6 | % | ||||||||
Research and Development Expenses
Research and development expenses decreased by $668,000 for the year ended December 31, 2024 compared to the prior year, primarily due to a decrease in project materials costs, partially offset by an increase in scientific research costs.
Research and development expenses relate to our continued development and production of our most advanced TriKE® product candidates GTB-3650 and GTB-5550 along with the progression on other promising candidates. In late June 2024, we received clearance from the FDA with respect to our IND Application in relation to GTB 3650, and we started study enrollment targeting patients with relapsed/refractory AML and high grade MDS on January 21, 2025. We anticipate our direct clinical and preclinical expenses to increase in 2025 as our next generation GTB-3650 camelid nanobody product is in the clinic. We also plan to complete the product development of GTB-5550 in 2025. We do not, however, anticipate an increase in related R&D licensing and administrative costs.
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses increased by approximately $3.4 million for the year ended December 31, 2024 compared to the prior year, primarily due to an increase in legal fees and settlement expenses.
Other Income (Expense)
| Years Ended December 31, | ||||||||||||||||
| 2024 | 2023 | $ Change | % Change | |||||||||||||
| Other Income (Expense): | ||||||||||||||||
| Interest income | $ | 402,000 | $ | 780,000 | $ | (378,000 | ) | (48 | )% | |||||||
| Interest expense | — | (213,000 | ) | 213,000 | (100 | )% | ||||||||||
| Change in fair value of warrant liability | 800,000 | 4,797,000 | (3,997,000 | ) | (83 | )% | ||||||||||
| Gain on extinguishment of debt | — | 547,000 | (547,000 | ) | (100 | )% | ||||||||||
| Unrealized gain (loss) on marketable securities | — | 48,000 | (48,000 | ) | (100 | )% | ||||||||||
| Other | — | 20,000 | (20,000 | ) | (100 | )% | ||||||||||
| Total Other Income (Expense) | $ | 1,202,000 | $ | 5,979,000 | $ | (4,777,000 | ) | (80 | )% | |||||||
| 34 |
Interest Income
Interest income decreased by $378,000 for the year ended December 31, 2024 compared to the prior year primarily due to lower short-term investment balances.
Interest Expense
Interest expense decreased by $213,000 for the year ended December 31, 2024 compared to the prior year as financing costs incurred associated with warrants accounted as warrant liability were incurred in the prior year but not in the current year.
Change in Fair Value of Warrant Liability
The change in fair value of warrant liability decreased by approximately $4.0 million for the year ended December 31, 2024 compared to the prior year, primarily due to the decline in the Company’s stock price at December 31, 2024, as compared to the prior year.
Gain on Extinguishment of Debt
Gain on extinguishment of debt decreased by $547,000 for the year ended December 31, 2024 compared to the prior year as the gain on extinguishment of debt that resulted from share settlements of a greater amount of vendor accounts payable than the fair value of the shares on the date of settlement occurred in the prior year but not in the current year.
Net Loss
| Years Ended December 31, | ||||||||||||||||
| 2024 | 2023 | $ Change | % Change | |||||||||||||
| Net Loss | $ | (13,162,000 | ) | $ | (7,597,000 | ) | $ | (5,565,000 | ) | 73 | % | |||||
Net loss increased $5,565,000 for the year ended December 31, 2024, primarily due to the decrease in the change in fair value of warrant liability, and an increase in legal fees, and was partially offset by a decrease in stock compensation and research and development expenses, all as described above.
Liquidity and Capital Resources
The accompanying financial statements have been prepared assuming that we will continue as a going concern. We do not have any product candidates approved for sale and have not generated any revenue from our product sales. We have sustained operating losses since inception, and we expect such losses to continue into the foreseeable future. Historically, we have financed our operations through public and private sales of common stock, issuance of preferred and common stock, issuance of convertible debt instruments, and strategic collaborations. For the year ended December 31, 2024, we recorded a net loss of approximately $13.2 million and used cash in operations of approximately $12.9 million. These factors raise substantial doubt about our ability to continue as a going concern within one year of the date that the financial statements are issued.
The financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern. Accordingly, the financial statements have been prepared on a basis that assumes we will continue as a going concern, and which contemplates the realization of assets and satisfaction of liabilities and commitments in the ordinary course of business.
We have evaluated the significance of the uncertainty regarding our financial condition in relation to our ability to meet our obligations, which has raised substantial doubt about our ability to continue as a going concern. While it is very difficult to estimate our future liquidity requirements we believe if we are unable to obtain additional financing, existing cash resources will not be sufficient to enable us to fund the anticipated level of operations through one year from the date the accompanying financial statements are issued. There can be no assurances that we will be able to secure additional financing on acceptable terms. In the event that we do not secure additional financing, we will be forced to delay, reduce, or eliminate some or all of our discretionary spending, which could adversely affect our business prospects, ability to meet long-term liquidity needs and ability to continue operations.
Cash Flows
| Years Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Statements of Cash Flow Data: | ||||||||
| Net cash used in operating activities | $ | (12,904,000 | ) | $ | (8,852,000 | ) | ||
| Net cash provided by (used in) investing activities | 12,893,000 | (2,009,000 | ) | |||||
| Net cash provided by financing activities | 2,976,000 | 6,268,000 | ||||||
| Net increase (decrease) in cash and cash equivalents and restricted cash | 2,965,000 | (4,593,000 | ) | |||||
| Cash and cash equivalents and restricted cash, beginning of period | 1,079,000 | 5,672,000 | ||||||
| Cash and cash equivalents and restricted cash, end of period | $ | 4,044,000 | $ | 1,079,000 | ||||
| 35 |
Operating Activities
Net cash used in operating activities was approximately $12.9 million for the year ended December 31, 2024, and was primarily due to a net loss of approximately $13.2 million, and a decrease in the fair value of warrant liability of $0.8 million.
Net cash used in operating activities was approximately $8.9 million for the year ended December 31, 2023, and was primarily due to a net loss of approximately $7.6 million, a decrease in the fair value of warrant liability of $4.8 million, and partially offset by stock compensation of $2.2 million and an increase in accounts payable and accrued expenses of approximately $2.0 million.
Investing Activities
Net cash provided (used) in financing activities for the years ended December 31, 2024 and 2023, resulted primarily from proceeds from the sale, or (purchase), of short-term investments.
Financing Activities
Net cash provided by financing activities for the years ended December 31, 2024 and 2023, resulted from proceeds from the issuance of common stock and warrants.
Working Capital (Deficit)
The following table summarizes total current assets, liabilities, and working capital (deficit) for the years ended December 31, 2024 and 2023:
| As of December 31 | ||||||||||||
| 2024 | 2023 | Increase/(Decrease) | ||||||||||
| Current assets | $ | 4,232,000 | $ | 14,056,000 | $ | (9,824,000 | ) | |||||
| Current liabilities | $ | 5,902,000 | $ | 6,633,000 | $ | (731,000 | ) | |||||
| Working capital (deficit) | $ | (1,670,000 | ) | $ | 7,423,000 | $ | (9,093,000 | ) | ||||
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements as of December 31, 2024.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
This company qualifies as a smaller reporting company, as defined in 17 C.F.R. §229.10(f) (1) and is not required to provide information by this Item.
| 36 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Stockholders and Board of Directors of GT Biopharma, Inc.
San Francisco, California
Opinion on the Financial Statements
We have audited the accompanying balance sheets of GT Biopharma, Inc. (the “Company”) as of December 31, 2024 and 2023 and the related statements of operations, changes in stockholders’ equity (deficit), and cash flows for the years then ended, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for the years then ended, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the financial statements, during the year ended December 31, 2024, the Company incurred a net loss of $13.2 million, used cash in operations of $12.9 million, and at December 31, 2024, had a stockholders’ deficit of $1.7 million. These matters raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matter Description
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that was communicated or required to be communicated to the audit committee and that (1) relates to accounts or disclosures that are material to the financial statements and (2) involved especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinions on the critical audit matter or on the accounts or disclosures to which they relate.
Valuation of Warrant Liability
Description of the Matter
As described in Note 5 to the financial statements, during the year ended December 31, 2023, the Company issued certain warrants to acquire its common stock and such warrants contained provisions and terms that resulted in the warrants requiring recognition as fair value liabilities. The warrant liabilities are required to be measured at fair value initially at issuance, and subsequently thereafter at each reporting date including December 31, 2024.
We identified auditing the valuation of the warrant liabilities as a critical audit matter due to the complexity of the accounting for the transaction and the significant judgements used by the Company in determining the fair value of the warrant liabilities. This required a high degree of auditor judgment and increased auditor effort in auditing the determination and valuation of the warrant liabilities.
| 37 |
How We Addressed the Matter in Our Audit
The primary procedures we performed to address this critical audit matter included:
| ● | We obtained and examined the warrant liability agreement, including assessing the reasonableness of its presentation as a liability in the financial statements. | |
| ● | We evaluated the appropriateness of the model used to value the warrant liability and tested the reasonableness of the assumptions used by the Company in determining the fair value of the warrant liability. | |
| ● | We developed an independent expectation of the warrant liability and compared our independent expectation to the Company calculated value. |
Commitments and Contingencies
Description of the Matter
As described in Note 8 to the financial statements, the Company is involved in certain legal proceedings that arise from time to time in the ordinary course of our business. The Company records accruals for contingencies to the extent that its management concludes that the occurrence is probable and that the related amounts of loss can be reasonably estimated.
We identified auditing the accruals of potential liability of these matters as a critical audit matter due to the significant judgements used by Management in determining the probable outcome of these matters. This required a high degree of auditor judgment and increased auditor effort in evaluating Managements’ assessment of the probability of outcome and disclosure of these matters.
How We Addressed the Matter in Our Audit
The primary procedures we performed to address this critical audit matter included:
| ● | We discussed the nature of these matters with Management, obtained and examined legal filings and other correspondence related to these matters, and circulated and examined legal confirmations to attorneys handling these matters. | |
| ● | We evaluated the appropriateness of the methodology used by Management in their assessment of any potential liability related to these matters | |
| ● | We developed an independent expectation of any potential liability and compared our independent expectation to the Company’s calculated value. |
PCAOB Firm ID: |
|
| February 21, 2025 |
| 38 |
GT BIOPHARMA, INC.
Balance Sheets
| December 31, 2024 | December 31, 2023 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | $ | ||||||
| Restricted cash | ||||||||
| Short-term investments | ||||||||
| Prepaid expenses and other current assets | ||||||||
| Total Current Assets | ||||||||
| Operating lease right-of-use asset | ||||||||
| TOTAL ASSETS | $ | $ | ||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
| Current liabilities | ||||||||
| Accounts payable | $ | $ | ||||||
| Accrued expenses | ||||||||
| Current operating lease liability | ||||||||
| Warrant liability | ||||||||
| Total Current Liabilities | ||||||||
| Stockholders’ Equity (Deficit) | ||||||||
| Convertible Preferred stock, par value $, shares authorized Series C - shares issued and outstanding at December 31, 2024 and 2023, respectively | ||||||||
| Common stock, par value $, shares authorized, and shares issued and outstanding as of December 31, 2024 and 2023, respectively | ||||||||
| Additional paid in capital | ||||||||
| Accumulated deficit | ( | ) | ( | ) | ||||
| Total Stockholders’ Equity (Deficit) | ( | ) | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | $ | $ | ||||||
The accompanying notes are an integral part of these financial statements.
| 39 |
GT BIOPHARMA, INC.
Statements of Operations
| For the Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Revenues | $ | $ | ||||||
| Operating Expenses: | ||||||||
| Research
and development (including $ | ||||||||
| Selling, general and administrative (including $ and $ of stock compensation granted to officers, directors, and employees and for services during the years ended December 31, 2024 and 2023, respectively) | ||||||||
| Loss from Operations | ( | ) | ( | ) | ||||
| Other Income (Expense) | ||||||||
| Interest income | ||||||||
| Interest expense | ( | ) | ||||||
| Change in fair value of warrant liability | ||||||||
| Gain on extinguishment of share settled debt | ||||||||
| Unrealized gain on short-term investments | ||||||||
| Other | ||||||||
| Total Other Income, Net | ||||||||
| Net Loss | $ | ( | ) | $ | ( | ) | ||
| Net Loss Per Share - Basic and Diluted | $ | ) | $ | ) | ||||
| Weighted average common shares outstanding - basic and diluted | ||||||||
The accompanying notes are an integral part of these financial statements.
| 40 |
GT BIOPHARMA, INC.
Statements of Stockholders’ Equity (Deficit)
| Preferred Shares | Common Shares | Additional Paid in | Accumulated | |||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | Total | ||||||||||||||||||||||
| Balance, December 31, 2022 | $ | $ | $ | $ | ( | ) | $ | |||||||||||||||||||||
| Private placement of common stock | - | |||||||||||||||||||||||||||
| Exercise of prefunded warrants for common stock | - | |||||||||||||||||||||||||||
| Initial recognition of fair value of warrant liability | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Issuance of common stock for services | - | |||||||||||||||||||||||||||
| Issuance of common stock to settle vendor payable | - | |||||||||||||||||||||||||||
| Fair value of vested stock options | - | - | ||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance, December 31, 2023 | ( | ) | ||||||||||||||||||||||||||
| Issuance of common stock and warrants for cash | - | |||||||||||||||||||||||||||
| Cancellation of common stock issued to prior CFO | - | ( | ) | |||||||||||||||||||||||||
| Issuance of common stock to settle vendor payable | - | |||||||||||||||||||||||||||
| Fair value of vested stock options | - | - | ||||||||||||||||||||||||||
| Net loss | - | - | ( | ) | ( | ) | ||||||||||||||||||||||
| Balance, December 31, 2024 | $ | $ | $ | $ | ( | ) | $ | ( | ) | |||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
| 41 |
GT BIOPHARMA, INC.
Statements of Cash Flows
| For the Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | ( | ) | $ | ( | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Stock based compensation – services | ||||||||
| Stock based compensation - officers, directors, and employees | ||||||||
| Change in fair value of warrant liability | ( | ) | ( | ) | ||||
| Gain on extinguishment of share settled debt | ( | ) | ||||||
| Unrealized gain on short-term investments | ( | ) | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Decrease in prepaid expenses | ( | ) | ( | ) | ||||
| Change in operating lease right-of-use assets | ||||||||
| Increase in accounts payable and accrued expenses | ||||||||
| (Decrease) in operating lease liability | ( | ) | ( | ) | ||||
| Net Cash Used in Operating Activities | ( | ) | ( | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Sale (Purchase) of short-term investments | ( | ) | ||||||
| Net Cash Provided by (Used in) Investing Activities | ( | ) | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of common stock and warrants, net | ||||||||
| Net Cash Provided by Financing Activities | ||||||||
| Net Increase (Decrease) in Cash and Cash Equivalents and Restricted Cash | ( | ) | ||||||
| Cash and Cash Equivalents and Restricted Cash at Beginning of Period | ||||||||
| Cash and Cash Equivalents and Restricted Cash at End of Period | $ | $ | ||||||
| SUPPLEMENTAL DISCLOSURES OF CASH FLOW INFORMATION: | ||||||||
| Cash paid during the year for: | ||||||||
| Interest | $ | $ | ||||||
| Income taxes | $ | $ | ||||||
| SUPPLEMENTAL DISCLOSURES OF NON-CASH INVESTING AND FINANCING ACTIVITIES | ||||||||
| Initial recognition of fair value of warrant liability | $ | $ | ||||||
| Fair value of common stock issued to a vendor to settle accounts payable | $ | $ | ||||||
The accompanying notes are an integral part of these financial statements.
| 42 |
GT Biopharma, Inc.
Notes to Financial Statements
For the Years Ended December 31, 2024 and 2023
Note 1 – Organization and Going Concern Analysis
Organization
The corporate predecessor of GT Biopharma, Inc, Diagnostic Data, Inc., was incorporated in the state of California in 1965. Diagnostic Data, Inc. changed its incorporation to the state of Delaware on December 21, 1972 and changed its name to DDI Pharmaceuticals, Inc. on March 11, 1985. On September 7, 1994, DDI Pharmaceuticals, Inc. merged with International BioClinical, Inc. and Bioxytech S.A. and changed its name to OXIS International, Inc. On July 17, 2017, OXIS International, Inc. changed its name to GT Biopharma, Inc.
Throughout this Annual Report on Form 10-K, the terms “GTBP,” “we,” “us,” “our,” “the Company” and “our Company” refer to GT Biopharma, Inc.
The GT Biopharma logo, TriKE®, and other trademarks or service marks of GT Biopharma, Inc. appearing in this quarterly report are the property of the Company. This Annual Report on Form 10-K also contains registered marks, trademarks and trade names of other companies. All other trademarks, registered marks and trade names appearing herein are the property of their respective holders.
The Company is a clinical stage biopharmaceutical company focused on the development and commercialization of novel immune-oncology products based on our proprietary Tri-specific Killer Engager (TriKE®), and Tetra-specific Killer Engager (Dual Targeting TriKE®) platforms. The Company’s TriKE® and Dual Targeting TriKE® platforms generate proprietary therapeutics designed to harness and enhance the cancer killing abilities of a patient’s own natural killer cells (NK cells).
Going Concern Analysis
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company does
not have any product candidates approved for sale and has not generated any revenue from its product sales. The Company has
sustained operating losses since inception, and expects such losses to continue into the foreseeable future. Historically, the
Company has financed its operations through public and private sales of common stock, issuance of preferred and common stock,
issuance of convertible debt instruments, and strategic collaborations. For the year ended December 31, 2024, the Company recorded a
net loss of approximately $
The financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern. Accordingly, the financial statements have been prepared on a basis that assumes the Company will continue as a going concern and which contemplates the realization of assets and satisfaction of liabilities and commitments in the ordinary course of business.
The Company has evaluated the significance of the uncertainty regarding the Company’s financial condition in relation to its ability to meet its obligations, which has raised substantial doubt about the Company’s ability to continue as a going concern. While it is very difficult to estimate the Company’s future liquidity requirements, the Company believes if it is unable to obtain additional financing, existing cash resources will not be sufficient to enable it to fund the anticipated level of operations through one year from the date the accompanying financial statements are issued. There can be no assurances that the Company will be able to secure additional financing on acceptable terms. In the event the Company does not secure additional financing, the Company will be forced to delay, reduce, or eliminate some or all of its discretionary spending, which could adversely affect the Company’s business prospects, ability to meet long-term liquidity needs and the ability to continue operations.
Note 2 – Summary of Significant Accounting Policies
Basis of Presentation and Principles of Consolidation
The accompanying financial statements have been prepared in accordance with the accounting principles generally accepted in the United States of America. The Company’s former wholly-owned subsidiaries, Oxis Biotech, Inc. and Georgetown Translational Pharmaceuticals, Inc., were both dissolved on October 22, 2024. Prior to their dissolution, the operations of Oxis Biotech, Inc. and Georgetown Translational Pharmaceuticals, Inc. was limited with insignificant assets and liabilities. The 2023 financial statements included the accounts of the Company and its former wholly-owned subsidiaries, Oxis Biotech, Inc. and Georgetown Translational Pharmaceuticals, Inc., and all intercompany transactions and balances were eliminated in consolidation.
| 43 |
Accounting Estimates
The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates include management’s estimates for continued liquidity, accruals for potential liabilities, assumptions used in deriving the fair value of warrant liabilities, valuation of equity instruments issued for debt and services and realization of deferred tax assets.
Cash Equivalents and Short-Term Investments
The
Company considers highly liquid investments with maturities of three months or less at the date of acquisition as cash equivalents in
the accompanying financial statements. At December 31, 2024 and 2023, total cash equivalents which consist of money market funds, amounted
to approximately $
Management
generally determines the appropriate classification of its investments at the time of purchase. We classify these investments as short-term
investments, as part of current assets, based upon our ability and intent to use any and all of these investments as necessary to satisfy
liquidity requirements that may arise from our business. Investments are carried at fair value with the unrealized holding gains and
losses reported in the accompanying statements of operations. At December 31, 2024 and 2023, total short-term investments
which consist of US treasuries and US government agencies, amounted to approximately $ and $
Restricted Cash
As of December 31, 2024, the Company has classified certain cash balances as restricted cash in its balance sheets. The Company’s restricted cash is deposited in a financial institution and held as a collateral for a credit card agreement with the same financial institution.
Fair Value of Financial Instruments
Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820-10 requires entities to disclose the fair value of financial instruments, both assets and liabilities recognized and not recognized on the balance sheet for which it is practicable to estimate fair value. ASC 820-10 defines the fair value of a financial instrument as the amount at which the instrument could be exchanged in a current transaction between willing parties.
The three levels of the fair value hierarchy are as follows:
Level 1 Valuations based on unadjusted quoted prices in active markets for identical assets or liabilities that the entity has the ability to access.
Level 2 Valuations based on quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable data for substantially the full term of the assets or liabilities.
Level 3 Valuations based on inputs that are unobservable, supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The carrying amounts of the Company’s other financial assets and liabilities, such as cash and cash equivalents, short-term investments, prepaid expenses and other current assets, accounts payable, accrued expenses, approximate their fair values because of the short maturity of these instruments.
The
carrying amount of the Company’s warrant liability of $
Warrant Liability
The Company evaluates its financial instruments to determine if such instruments are derivatives or contain features that qualify as embedded derivatives in accordance with ASC Topic 815, “Derivatives and Hedging” (“ASC 815”). For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the statements of operations.
The Company’s use of derivative financial instruments is generally limited to warrants issued by the Company that do not meet the criteria for equity treatment and are recorded as liabilities. We do not use financial instruments or derivatives for any trading purposes.
| 44 |
Common Stock (February 2024 Reverse Stock-Split)
As a result of the reverse stock-split, every thirty (30) shares of issued and outstanding common stock were automatically combined into one issued and outstanding share of common stock, without any change in the par value per share. No fractional shares will be issued in connection with the reverse stock-split. Stockholders who otherwise would be entitled to receive fractional shares of common stock will be entitled to receive their pro-rata portion of the net proceeds obtained from the aggregation and sale by the exchange agent of the fractional shares resulting from the reverse stock-split (reduced by any customary brokerage fees, commission and other expenses). The reverse stock-split reduced the number of shares of common stock outstanding on the effective date of the reverse stock-split from shares to shares, subject to minor adjustments due to the treatment of fractional shares. The number of authorized shares of common stock remains unchanged at shares.
Proportionate adjustments have been made to the per share exercise price and the number of shares of common stock that may be purchased upon exercise of outstanding stock options and warrants for the Company’s common stock, and to the number of shares of common stock reserved for future issuance pursuant to the Company’s 2022 Omnibus Incentive Plan.
All share and per share information within this report have been adjusted to retroactively reflect the reverse stock-split as of the earliest period presented.
Stock-Based Compensation
The Company periodically issues stock-based compensation to officers, directors, employees, and consultants for services rendered. Such issuances vest and expire according to terms established at the issuance date.
Stock-based payments to officers, directors, employees, and consultants in exchange for goods and services, which include grants of employee stock options, are recognized in the financial statements based on their grant date fair values in accordance with ASC 718, Compensation-Stock Compensation. Stock based payments to officers, directors, employees, and consultants, which are generally time vested, are measured at the grant date fair value and depending on the conditions associated with the vesting of the award, compensation cost is recognized on a straight-line or graded basis over the vesting period. Recognition of compensation expense for non-employees is in the same period and manner as if the Company had paid cash for the services. The fair value of stock options granted is estimated using the Black-Scholes option-pricing model, which uses certain assumptions related to risk-free interest rates, expected volatility, expected life, and future dividends. The assumptions used in the Black-Scholes option pricing model could materially affect compensation expense recorded in future periods.
Research and Development Expenses
Costs incurred for research and development are expensed as incurred. The salaries, benefits, and overhead costs of personnel conducting research and development of the Company’s products are included in research and development expenses. Purchased materials that do not have an alternative future use are also expensed.
Leases
The Company accounts for its lease in accordance with the guidance of ASC 842, Leases. The Company determines whether a contract is, or contains, a lease at inception. Right-of-use assets represent the Company’s right to use an underlying asset during the lease term, and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Right-of-use assets and lease liabilities are recognized at lease commencement based upon the estimated present value of unpaid lease payments over the lease term. The Company uses its incremental borrowing rate based on the information available at lease commencement in determining the present value of unpaid lease payments.
The Company’s lease expired in June 2024 and was not renewed, and the Company became a fully remote company.
Basic earnings (loss) per share is computed using the weighted-average number of common shares outstanding during the period. Diluted earnings (loss) per share is computed using the weighted-average number of common shares and the dilutive effect of contingent shares outstanding during the period. Potentially dilutive contingent shares, which primarily consist of stock issuable upon exercise of stock options and warrants, have been excluded from the diluted loss per share calculation because their effect is anti-dilutive.
| 45 |
| December 31, 2024 | December 31, 2023 | |||||||
| Options to purchase common stock | ||||||||
| Warrants to purchase common stock | ||||||||
| Total anti-dilutive securities | ||||||||
Concentration
Cash
is deposited in one financial institution. The balances held at this financial institution at times may be in excess of Federal Deposit
Insurance Corporation (“FDIC”) insurance limits of up to $
The Company has a significant concentration of expenses incurred from and accounts payable to Cytovance, a related party, and the University of Minnesota, see Note 4 – Accounts Payable and Related Party.
Segment Information
The Company’s Chief Executive Officer and President (“CEO”) is our chief operating decision maker (“CODM”) and evaluates performance and makes operating decisions about allocating resources based on financial data presented on a consolidated basis. Because our CODM evaluates financial performance on a consolidated basis, the Company has determined that it operates as a single reportable segment composed of the financial results of GT Biopharma, Inc. (see Note 9).
Recent Accounting Pronouncements
In November 2023, the FASB issued ASU 2023-07, “Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosure.” The amendments expand a public entity’s segment disclosures by requiring disclosure of significant segment expenses that are regularly provided to the chief operating decision maker, requiring other new disclosures, and requiring enhanced interim disclosures. ASU 2023-07 requires public entities with a single reportable segment to provide all the disclosures required by this standard and all existing segment disclosures in Topic 280 on an interim and annual basis. ASU 2023-07 is effective for annual periods beginning after December 15, 2023, and interim periods beginning after December 15, 2024, applied retrospectively with early adoption permitted. As of December 31, 2024, the Company has adopted ASU 2023-07. The adoption of this standard did not have a material impact on the Company’s financial statements but has resulted in additional disclosures within the footnotes to our financial statements (See Note 9).
The Company’s management has evaluated all the recently issued, but not yet effective, accounting standards and guidance that have been issued or proposed by the FASB or other standards-setting bodies through the filing date of these financial statements and does not believe the future adoption of any such pronouncements will have a material effect on the Company’s financial position and results of operations.
Note 3 – Fair Value of Financial Instruments
Financial Assets
The following table represents the estimated fair values of the Company’s financial instruments:
| December 31, 2023 | ||||||||||||||||
| Unrealized | Unrealized | Fair | ||||||||||||||
| Cost | Gains | Losses | Value | |||||||||||||
| Short-term investments | $ | $ | $ | $ | ||||||||||||
| Total | $ | $ | $ | $ | ||||||||||||
| 46 |
The following table represents the Company’s fair value hierarchy for its financial assets (cash equivalents and investments):
| December 31, 2024 | ||||||||||||||||
| Fair Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Cash equivalents: | ||||||||||||||||
| Money market funds | $ | $ | $ | $ | ||||||||||||
| US treasuries | ||||||||||||||||
| Short-term investments: | ||||||||||||||||
| US treasuries | ||||||||||||||||
| Total financial assets | $ | $ | $ | $ | ||||||||||||
| December 31, 2023 | ||||||||||||||||
| Fair Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Cash equivalents: | ||||||||||||||||
| Money market funds | $ | $ | $ | $ | ||||||||||||
| Short-term investments: | ||||||||||||||||
| US treasuries and US gov’t. agencies | ||||||||||||||||
| Total financial assets | $ | $ | $ | $ | ||||||||||||
Warrant Liability
For the details of warrant liability transactions see Note 5 – Warrant Liability.
Note 4 – Accounts Payable and Related Party
Accounts payable consists of the following:
| As of | As of | |||||||||||||||
| December 31, 2024 | % | December 31, 2023 | % | |||||||||||||
| Accounts payable to Cytovance, a related party 1 | $ | % | $ | % | ||||||||||||
| Accounts payable to University of Minnesota | % | % | ||||||||||||||
| Legal services firm | % | % | ||||||||||||||
| Other accounts payable | % | % | ||||||||||||||
| Total accounts payable | $ | $ | ||||||||||||||
| 1 | Accounts Payable to Cytovance Biologics, Inc. (“Cytovance”), a Related Party, since Cytovance owns greater than 5% of the Company’s issued and outstanding common stock. See Note 8 – Commitments and Contingencies, Significant Agreements. |
The details of the Company’s accounts payable to Cytovance Biologics, Inc., were as follows:
| Year Ending | ||||||||
| December 31, 2024 | December 31, 2023 | |||||||
| Beginning balance | $ | $ | ||||||
| Invoices, net | ||||||||
| Payments in cash | ( | ) | ( | ) | ||||
| Payments in common stock, at fair value | ( | ) | ( | ) | ||||
| Ending balance | $ | $ | ||||||
During
the year ended December 31, 2024, the Company issued shares of common stock with a fair value of $
During
the year ended December 31, 2023, the Company issued shares of common stock with a fair value of $
| 47 |
University of Minnesota
See Note 8 – Commitments and Contingencies, Significant Agreements.
Note 5 – Warrant Liability
2023 Warrants
On
January 4, 2023, as part of the private placement offering, the Company issued common stock, warrants to purchase up to an aggregate
of
The 2023 Common Warrants and the 2023 Placement Agents Warrants (collectively the “2023 Warrants”), provide for a value calculation for the warrants using the Black Scholes model in the event of certain fundamental transactions. The fair value calculation provides for a floor on the volatility amount utilized in the value calculation at % or greater. The Company has determined this provision introduces leverage to the holders that could result in a value that would be greater than the settlement amount of a fixed-for-fixed option on the Company’s own equity shares. Therefore, pursuant to ASC 815, the Company has classified the 2023 Warrants as a liability in its balance sheet. The classification of the 2023 Warrants, including whether they should be recorded as liability or as equity, is evaluated at the end of each reporting period.
The
2023 Warrants were initially recorded at a fair value at $
The 2023 Warrant liability is valued using a BlackScholes Option pricing model with the following assumptions:
| 2023 Warrants | ||||||||
| December 31, 2024 | December 31, 2023 | |||||||
| Stock price | $ | $ | ||||||
| Risk-free interest rate 1 | % | % | ||||||
| Expected volatility 2 | % | % | ||||||
| Expected life (in years) 3 | ||||||||
| Expected dividend yield 4 | ||||||||
| Fair value of warrants | $ | $ | ||||||
| 1 |
| 2 |
| 3 |
| 4 |
2020 Warrants
The Company issued warrants underlying shares of common stock during the year ended December 31, 2020 (the “2020 Warrants”), that contained a fundamental transaction provision that could give rise to an obligation to pay cash to the warrant holder upon occurrence of certain change in control type events. In accordance with ASC 480, the fair value of the 2020 Warrants is classified as a liability in the Company’s balance sheets and will be re-measured at the end of every reporting period with the change in value reported in the statements of operations until they are either exercised or expire.
The 2020 Warrant liability is valued using a Binomial pricing model with the following assumptions:
| 2020 Warrants | ||||||||
| December 31, 2024 | December 31, 2023 | |||||||
| Stock price | $ | $ | ||||||
| Risk-free interest rate 1 | % | % | ||||||
| Expected volatility 2 | % | % | ||||||
| Expected life (in years) 3 | ||||||||
| Expected dividend yield 4 | ||||||||
| Fair value of warrants | $ | $ | ||||||
At December 31, 2024, the estimated fair value of these 2020 Warrants was de minimis, as such, none was recorded.
| 48 |
Warrant Liability
The details of warrant liability transactions were as follows:
| Year Ending | ||||||||
| December 31, 2024 | December 31, 2023 | |||||||
| Beginning balance | $ | $ | ||||||
| Issuance of warrants at fair value | ||||||||
| Change in fair value | ( | ) | ( | ) | ||||
| Extinguishment | ||||||||
| Ending balance | $ | $ | ||||||
Note 6 – Stockholders’ Equity (Deficit)
The Company’s authorized capital as of December 31, 2024 was shares of common stock, par value $ per share, and shares of preferred stock, par value $ per share.
Common Stock
2024 Common Stock Offering
On
May 23, 2024, the Company received gross proceeds of approximately $
Pursuant to the Purchase Agreement, the Company agreed for a period of one year following the closing date of the offering not to (i) issue or agree to issue equity or debt securities convertible into, or exercisable or exchangeable for, shares at a conversion price, exercise price or exchange price which floats with the trading price of the 2024 Shares or which may be adjusted after issuance upon the occurrence of certain events or (ii) enter into any agreement, including an equity line of credit, whereby the Company may issue securities at a future-determined price.
The Company determined that under ASC 815, the 2024 Common Warrants and the 2024 Placement Agent Warrants are considered indexed to the Company’s own stock and eligible for an exception from derivative accounting. Accordingly, the fair value of the 2024 Common Warrants and the 2024 Placement Agent Warrants are classified as equity.
2023 Private Placement of Common Stock
On
January 4, 2023, the Company received gross proceeds of $
| 49 |
The 2023 Common Warrants and the 2023 Placement Agents Warrants contained a clause not considered to be within the Company’s control and was accounted as a warrant liability (see Note 5 – Warrant Liability).
Common Stock Issued for Services
During
the year ended December 31, 2023, the Company issued
shares of common stock with a fair value of $
Preferred Stock
Series C Preferred Stock
As of December 31, 2024 and 2023, there were shares of series C preferred stock, par value $ per share (the “Series C Preferred Stock”) issued and outstanding.
As a result of numerous reverse stock-splits in previous years and the agreement terms for adjusting the rights of the related shares, the shares of Series C Preferred Stock are convertible into an infinitesimal amount of common stock, have no voting rights, and in the event of liquidation, the holders of the Series C Preferred Stock would not participate in any distribution of the assets or surplus funds of the Company. The holders of Series C Preferred Stock also are not currently entitled to any dividends if and when declared by the Board. No dividends to holders of the Series C Preferred Stock were declared or unpaid through December 31, 2024 and 2023, respectively.
Series K Preferred Stock
On February 16, 2021, the Board designated shares of Series K preferred stock, par value $ (the “Series K Preferred Stock”).
Shares of the Series K Preferred Stock are convertible at any time, at the option of the holders, into shares of the Company’s common stock at an effective conversion rate of shares of common stock for each share of Series K Preferred. Shares of the Series K Preferred Stock have the same voting rights as the shares of the Company’s common stock, with the holders of the Series K Preferred Stock entitled to vote on an as-converted-to-common stock basis, subject to the beneficial ownership limitation, together with the holders of the Company’s common stock on all matters presented to the Company’s stockholders. The Series K Preferred Stock are not entitled to any dividends (unless specifically declared by the Board) but will participate on an as-converted-to-common-stock basis in any dividends to the holders of the Company’s common stock. In the event of the Company’s dissolution, liquidation or winding up, the holders of the Series K Preferred Stock will be on parity with the holders of the Company’s common stock and will participate, on a on an as-converted-to-common stock basis, in any distribution to holders of the Company’s common stock.
As of December 31, 2024 and 2023, there were shares of Series K Preferred stock issued and outstanding.
Note 7 – Common Stock Warrants and Options
Common Stock Warrants
Stock warrant transactions for the years ended December 31, 2024 and 2023, were as follows:
| Number of | Weighted Average | |||||||
| Warrants | Exercise Price | |||||||
| Warrants outstanding at December 31, 2022 | $ | |||||||
| Granted | ||||||||
| Forfeited/cancelled | ( | ) | ||||||
| Exercised | ( | ) | ||||||
| Warrants outstanding at December 31, 2023 | $ | |||||||
| Granted | ||||||||
| Forfeited/cancelled | ( | ) | ||||||
| Exercised | ||||||||
| Warrants outstanding at December 31, 2024 | $ | |||||||
| Warrants exercisable at December 31, 2024 | $ | |||||||
| 50 |
As of December 31, 2024, all outstanding warrants are fully vested and had an exercise price greater than the market price of the Company’s common stock, which resulted in intrinsic value.
Warrants outstanding as of December 31, 2024, 2024 are exercisable as follows:
| Warrants Outstanding and Exercisable as of September 30, 2024 | ||||||||||||||
Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | |||||||||||
| $ | $ | |||||||||||||
Common Stock Options
In April 2022 the Company established the 2022 Omnibus Incentive Plan (the “Plan”). The Plan was approved by our Board and stockholders. The purpose of the Plan is to grant stock and options to purchase our common stock, and other incentive awards, to our employees, directors, and key consultants. The maximum number of shares of common stock that may be issued pursuant to awards granted under the Plan is . The shares of our common stock underlying cancelled and forfeited awards issued under the Plan may again become available for grant under the Plan. As of December 31, 2024, there were stock options outstanding and shares of restricted stock granted in prior years under the Plan, which left shares available for grant under the Plan.
| Number of | Weighted Average | |||||||
| Options | Exercise Price | |||||||
| Options outstanding at December 31, 2022 | $ | |||||||
| Granted | ||||||||
| Forfeited/cancelled | ( | ) | ||||||
| Exercised | ||||||||
| Options outstanding at December 31, 2023 | $ | |||||||
| Granted | ||||||||
| Forfeited/cancelled | ( | ) | ||||||
| Exercised | ||||||||
| Options outstanding at December 31, 2024 | $ | |||||||
| Options exercisable at December 31, 2024 | $ | |||||||
The weighted average remaining contractual life of all options outstanding, and all options vested and exercisable as of December 31, 2024 was years. Furthermore, as of December 31, 2024, the intrinsic value of options outstanding amounted to approximately $, and the intrinsic value of options vested and exercisable amounted to approximately $.
| 51 |
On October 17, 2024, the Company granted stock options to an officer to purchase an aggregate of shares of common stock. The stock options are exercisable at $ per share, expire in years, vest over three years with a fair value of approximately $ on the date of grant which will be amortized over the vesting period.
In January and May 2023, the Company granted stock options to a employees and members of the Board to purchase shares of common stock. The stock options are exercisable at $ and $ per share, expire in years, vest over twelve months with a fair value of $ million on the date of grant which will be amortized over the vesting period.
The total fair value of options that vested during years ended December 31, 2024 and 2023, was $ and $, respectively, and is included in selling, general and administrative expense in the accompanying statements of operations. As of December 31, 2024, stock options were vested and exercisable and unvested compensation expense amounted to approximately $.
Range of Exercise Price | Number Outstanding | Weighted Average Remaining Contractual Life (Years) | Weighted Average Exercise Price | |||||||||||
| $ | $ | |||||||||||||
Note 8 – Commitments and Contingencies
Litigation
The Company is involved in certain legal proceedings that arise from time to time in the ordinary course of our business. Except for income tax contingencies, we record accruals for contingencies to the extent that our management concludes that the occurrence is probable and that the related amounts of loss can be reasonably estimated. Legal expenses associated with the contingency are expensed as incurred. There is no current or pending litigation of any significance with the exception of the matters identified below that have arisen under, and are being handled in, the normal course of business:
Ohri Matter
On July 22, 2024, the Company filed an AAA Arbitration Demand against Manu Ohri, its former Chief Financial Officer. In the Demand, the Company asserts claims against Mr. Ohri for breach of his fiduciary duties and breach of contract and seeks a declaratory judgment providing that the Company may characterize Mr. Ohri’s termination as “for cause” under his Employment Agreement, and that the Company may revoke the Separation Agreement entered into between the Company and Mr. Ohri prior to the Company learning of Mr. Ohri’s breaches. In addition to the declaratory judgment, the Company seeks damages arising from Mr. Ohri’s violations, and attorneys’ fees and any forum and arbitration fees. On September 3, 2024, Mr. Ohri filed both a general denial of the Company’s claims against him and counterclaims for breach of his Employment Agreement and Separation Agreement. A final hearing is scheduled for June 10th through June 12th, 2025 in Los Angeles, California. At this stage in the proceedings the Company is not able to determine the probability of the outcome of this matter or a range of reasonably expected losses, if any. The Company believes it has recorded an appropriate accrual for the matter.
Berk Matter
On November 14, 2023, former interim Chief Executive Officer, Dr. Gregory Berk filed a lawsuit in the US District Court for the District of Massachusetts alleging that the Company discriminated and retaliated against Dr. Berk for engaging in protected whistleblowing activity in violation of the Sarbanes Oxley Act (“SOX”). Although the Company vigorously defended this matter and believes it to be without merit, the lawsuit was dismissed with prejudice on December 4, 2024 as a result of the parties’ settlement. The Company has recorded the monetary settlement for this matter in the accompanying financial statements.
| 52 |
TWF Global Matter
On May 24, 2023, TWF Global, LLC (“TWF”) filed a Complaint in the California Superior Court for the County of Los Angeles naming the Company as defendant. The Complaint alleges that TWF is the holder of two Convertible Promissory Notes (“Notes”) and that the Company did not deliver shares of common stock due on conversion in February 2021. TWF was seeking per diem liquidated damages based on the terms of alleged Notes. On July 14, 2023, the Company filed a motion to dismiss for improper forum because the terms of the Notes, as alleged, require disputes to be filed in New York state and federal courts. TWF voluntarily dismissed its Complaint before the California Superior Court of Los Angeles without prejudice. The Company subsequently filed a Summons and Complaint for Interpleader against TWF and Z-One LLC before the Supreme Court of the State of New York County of New York, asking the Supreme Court to determine if the Company’s shares of common stock are properly registered to TWF or Z-One LLC, as both of these entities have made conflicting demands for registration of the shares of common stock. On February 5, 2024, the Company filed a motion for entry of default against TWF, seeking an order directing the Company to register the shares of common stock in the name of Z-One and that the Company be released from all associated liability and claims. The Court denied the motion without prejudice and agreed to reconsider the motion without further briefing upon the filing of a supplemental party affidavit. On May 9, 2024, Z-One filed a motion for summary judgement seeking dismissal of the action, representing that Z-One and TWF have settled their dispute over the entitlement to the Company’s shares of common stock and there is no remaining dispute before the Court. On May 21, 2024, the Company filed a supplemental affidavit in support of its motion for entry of default. On November 14, 2024, the Court held a hearing on the parties’ motions, at which the Court found that the motion for entry of default was mooted by the settlement agreement between Z-One and TWF. The Court ordered that the case be dismissed. On February 17, 2025, Z-One, LLC filed a Summons with Notice in the Supreme Court of the State of New York, County of New York. Z-One alleges that it was assigned TWF’s rights under the Notes and seeks enforcement and damages. The Company intends to demand a complaint, and seek dismissal of the case. The Company believes that the claims related to the Notes are without merit, and will continue to vigorously defend against these claims.
Handelman Matter
On
May 13, 2022, the Company made an arbitration demand upon Michael Handelman, its former Chief Financial Officer, asserting that he breached
his fiduciary duty by misappropriating Company funds and shares of common stock, among other things. The Company sought among other relief,
monetary damages estimated at $
Significant Agreements
Cytovance Biologics, Inc., a Related Party
In October 2020, the Company entered into a Master Services Agreement with Cytovance Biologics, Inc. (“Cytovance”), to perform biologic development and manufacturing services, and to produce and test compounds used in the Company’s potential product candidates. The Company subsequently executed numerous Statements of Work (“SOWs”) for the research and development of products for use in clinical trials.
On
August 24, 2022, the Company entered into a Settlement and Investment Agreement with Cytovance that amended existing SOWs and allowed
for future invoices to be settled in in a combination of cash and issuance of the Company’s common stock. The Agreement also set
Cytovance’s beneficial ownership limitation at
On
April 25, 2024, the Company entered into an Amendment to the Settlement and Investment Agreement with Cytovance that increased Cytovance’s
beneficial ownership limitation to
During
the years ended December 31, 2024 and 2023, the Company recognized research and development expenses of $
On
June 30, 2024, Cytovance became a related party as their beneficial ownership exceeded
As
of December 31, 2024 the Company’s commitments in relation to unbilled and unaccrued SOWs and any related Change Orders from Cytovance
for services that have not yet been rendered as of December 31, 2024, amounted to approximately $
| 53 |
University of Minnesota
2021 Scientific Research Agreement
Effective
June 16, 2021, the Company entered into a scientific research agreement with the Regents of the University of Minnesota, expiring on
June 30, 2023. Payments totaling approximately $
The
Company recorded an expense classified as research and development of approximately $
As of December 31, 2024 there were no outstanding commitments in relation to unbilled and unaccrued amounts from the University of Minnesota pursuant to the 2021 Scientific Research Agreement for services that have not yet been rendered as of December 31, 2024,.
2023 Sponsored Research Agreement
On
May 20, 2024, the Company entered into a sponsored research agreement with the Regents of the University of Minnesota (the
“2023 Sponsored Research Agreement”), effective July 1, 2023, and expiring on July 1, 2025. Payments totaling
approximately $
The
Company recorded an expense classified as research and development of approximately $
As
of December 31, 2024 the Company’s commitments in relation to unbilled and unaccrued amounts from the University of Minnesota pursuant
to the 2023 Sponsored Research Agreement for services that have not yet been rendered as of December 31, 2024, amounted to approximately
$
2016 Exclusive Patent License Agreement
Effective
July 18, 2016, the Company entered into an exclusive patent license agreement with the Regents of the University of Minnesota (as amended,
the “2016 Exclusive Patent License Agreement”), to further develop and commercialize cancer therapies using TriKE®
technology developed by researchers at the University of Minnesota to target NK cells to cancer. Under the terms of the agreement,
the Company receives exclusive rights to conduct research and to develop, make, use, sell, and import TriKE® technology
worldwide for the treatment of any disease, state, or condition in humans. The Company is responsible for obtaining all permits, licenses,
authorizations, registrations, and regulatory approvals required or granted by any governmental authority anywhere in the world that
is responsible for the regulation of products such as the TriKE® technology, including without limitation the FDA and
the European Agency for the Evaluation of Medicinal Products in the European Union. The agreement requires an upfront payment of $
Effective
May 13, 2024, the Company entered into an amended and restated exclusive patent license agreement with the Regents of the University
of Minnesota. The amendment requires an upfront payment of $
The
Company recorded an expense classified as research and development of $
| 54 |
2021 Exclusive License Agreement
Effective
March 26, 2021, the Company entered into an exclusive license agreement with the Regents of the University of Minnesota (the “2021
Exclusive Patent License Agreement”), specific to the B7H3 targeted TriKE®. The agreement requires an upfront payment
of $
The Company did not incur any expenses pursuant to the 2021 Exclusive License Agreement, for years ended December 31, 2024 and 2023, respectively.
2024 GTB-3650 Clinical Trial Agreement
On
November 18, 2024, the Registrant entered into an Investigator Initiated Clinical Trial Agreement (the “Agreement”) with
the Regents of the University of Minnesota (the “University”), pursuant to which, the University shall sponsor an Investigational
New Drug (“IND”) application for IND 165546 GTB-3650 (the “Research Program”) and shall serve as a sponsor investigator
for a phase 1 clinical trial entitled, “GTB-3650 (CD16/IL-15/CD33) Tri-Specific Killer Engager (TriKE) for the Treatment of High
Risk Myelodysplastic Syndromes (MDS), Refractory/Relapsed Acute Myeloid Leukemia (AML), and Minimal Residual Disease in AML,” designed
by the University (the “Study”). The Research Program is being conducted for clinical research use. The budget for the Study,
including without limitations, funding and resources, provides for up to approximately $
The
Company recorded an expense classified as research and development of approximately $
As
of December 31, 2024 the Company’s commitments in relation to unbilled and unaccrued amounts from the University of Minnesota pursuant
to the 2024 Clinical Trial Agreement for services that have not yet been rendered as of December 31, 2024, amounted to approximately
$
Contingency
On
November 21, 2024, the Company received a letter from Nasdaq notifying the Company that its amount of stockholders’ equity has
fallen below the $
Nasdaq’s Letter has no immediate effect on the listing of the Company’s common stock, and its common stock will continue to trade on The Nasdaq Capital Market under the symbol “GTBP” at this time. Pursuant to Nasdaq Listing Rules, the Company provided Nasdaq with a plan to achieve and sustain compliance on December 31, 2024. If Nasdaq accepts the Company’s plan to regain compliance, Nasdaq may grant an extension of up to 180 calendar days from the date of the Letter to evidence compliance. If Nasdaq does not accept the Company’s plan to regain compliance, the Company will have the opportunity to appeal the decision to a Nasdaq Hearings Panel. The Company intends to submit to Nasdaq, within the requisite time period, a plan to regain compliance with Listing Rule 5550(b)(1). There can be no assurance that Nasdaq will accept the Company’s plan, that the Company will be able to regain compliance with Listing Rule 5550(b)(1) or that the Company will be able meet the continued listing requirements during any compliance period that may be granted by Nasdaq.
In the future, if we fail to maintain such minimum requirements and a final determination is made by Nasdaq that our common stock must be delisted, the liquidity of our common stock would be adversely affected and the market price of our common stock could decrease. In addition, if delisted, we would no longer be subject to Nasdaq rules, including rules requiring us to have a certain number of independent directors and to meet other corporate governance standards. Our failure to be listed on Nasdaq or another established securities market would have a material adverse effect on the value of your investment in us.
Note 9 – Segment Information
The Company operates and manages its business as one reportable and operating as a clinical stage biopharmaceutical company focused on the development and commercialization of novel immune-oncology products based on our proprietary Tri-specific Killer Engager (TriKE®), and Tetra-specific Killer Engager (Dual Targeting TriKE®) platforms. The measure of segment assets is reported on the balance sheet as total assets.
The Company’s CODM reviews financial information presented and decides how to allocate resources based on net income (loss). Net income (loss) is used for evaluating financial performance.
Significant segment expenses include research and development, salaries, insurance, and stock-based compensation. Operating expenses include all remaining costs necessary to operate our business, which primarily include external professional services and other administrative expenses. The following table presents the significant segment expenses and other segment items regularly reviewed by our CODM:
| Year Ended December 31, | ||||||||
| 2024 | 2023 | |||||||
| Research and development | $ | $ | ||||||
| Salaries | ||||||||
| Insurance | ||||||||
| Stock-based compensation | ||||||||
| Operating expenses | ||||||||
| Other (income) expense | ( | ) | ||||||
| Net loss | $ | $ | ||||||
Note 10 – Income Tax
The
Company did not record any income tax provision for the years ended December 31, 2024 and 2023, respectively, due to the Company’s
net losses. The Company files income tax returns in the United States (“Federal”) and California, Minnesota and Massachusetts
(“State”) jurisdictions. The Company is subject to Federal and State income tax examinations by tax authorities for all years
since its inception. At December 31, 2024, the Company had Federal and State net operating loss carry forwards available to offset future
taxable income of approximately $
| 55 |
Based
on the weight of available evidence, including cumulative losses in recent years and expectations of future taxable income, the Company
has determined that it was more likely than not that its deferred tax assets would not be realized at December 31, 2024 and 2023, respectively.
Accordingly, the Company has recorded a valuation allowance for
Deferred taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes, and operating losses and tax credit carryforwards. The significant components of net deferred income tax assets are:
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| Deferred tax assets: | ||||||||
| Federal net operating loss carryforward | $ | $ | ||||||
| Stock based compensation and other items | ||||||||
| Intellectual property | ||||||||
| Section 14 research and development | ||||||||
| Deferred tax assets before valuation | ||||||||
| Valuation allowance | ( | ) | ( | ) | ||||
| Net deferred income tax assets | $ | $ | ||||||
A reconciliation of the federal statutory income tax rate and the effective income tax rate as a percentage of income before income tax provision is as follows for the year ended:
| December 31, | ||||||||
| 2024 | 2023 | |||||||
| Federal statutory income tax rate | % | % | ||||||
| State tax, net of federal benefit | % | % | ||||||
| Change in valuation allowance on net operating loss carryforwards | ( | )% | ( | )% | ||||
| Effective income tax rate | % | % | ||||||
| 56 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
There were no changes in or disagreements with our accountants on accounting and financial disclosure during the last two fiscal years.
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and Chief Financial Officer, evaluated the effectiveness of our disclosure controls and procedures as of the end of the period covered by this Annual Report on Form 10-K. For purposes of this section, the term disclosure controls and procedures means controls and other procedures of an issuer that are designed to ensure that information required to be disclosed by the issuer in the reports that it files or submits under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of December 31, 2024, the end of the period covered by this report, our disclosure controls and procedures were effective at a reasonable assurance level.
| 57 |
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of, a company’s principal executive and principal accounting officers and effected by a company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of the financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| ● | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the Company | |
| ● | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of the financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and | |
| ● | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
All internal control systems, no matter how well designed, have inherent limitations and can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within our company have been detected. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Management evaluated the effectiveness of our internal control over financial reporting as of December 31, 2024, using the framework set forth in the report of the Treadway Commission’s Committee of Sponsoring Organizations (“COSO”),“2013 Internal Control– Integrated Framework.” Based upon that evaluation, management believes our internal control over financial reporting was effective as of December 31, 2024.
Inherent Limitations on the Effectiveness of Controls
Management does not expect that our disclosure controls and procedures or our internal control over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control systems are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in a cost-effective control system, no evaluation of internal control over financial reporting can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been or will be detected.
These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of a simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Changes in Internal Controls Over Financial Reporting
Management has evaluated, with the participation of our Chief Executive Officer and Chief Financial Officer, whether any changes in our internal control over financial reporting that occurred during our last fiscal year have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting. Based on the evaluation we conducted, management has concluded that no such changes have occurred.
ITEM 9B. OTHER INFORMATION
ITEM 9C. DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS
Not applicable.
| 58 |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The following table sets forth the name, age, position and date of appointment of each of our directors and executive officers as of February 19, 2025.
| Name | Age | Position | Date of Appointment | |||
| Michael Breen | 62 | Executive Chairman of the Board and Interim Chief Executive Officer |
January 13, 2021 | |||
| Alan Urban | 56 | Chief Financial Officer & Secretary | June 3, 2024 | |||
| Bruce Wendel(1) (4) | 71 | Vice Chairman of the Board | November 11, 2020 | |||
| Rajesh Shrotriya, M.D.(2) (4) | 80 | Director | January 13, 2021 | |||
| Charles J. Casamento.(3) (4) | 79 | Director | May 1, 2023 |
| (1) | Chairman of the Compensation Committee. | |
| (2) | Chairman of the Nominating and Corporate Governance Committee. | |
| (3) | Chairman of the Audit Committee. | |
| (4) | Member of the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee. |
Michael Breen – Executive Chairman of the Board and Interim Chief Executive Officer
Mr. Breen was appointed to our Board of Directors on January 13, 2021, was appointed Executive Chairman of the Board on November 8, 2021 and was appointed as our Interim Chief Executive Officer on March 2, 2022. Prior to joining our company, Mr. Breen served as a senior partner in the global law firm of Clyde & Co., specializing in all aspects of corporate law, including mergers and acquisitions and fund management regulatory issues, which included advising clients in the biotechnology and health sciences sectors. Prior to joining Clyde & Co., Mr. Breen served as a senior partner and managing partner in the London law firm of Edward Lewis. Prior to his time at Edward Lewis, he was also a partner at Robert Gore & Company. Between 2002 and 2005, Mr. Breen was managing director and a shareholder of the Sports and Entertainment Division of Insinger de Beaufort Bank, a Dutch private banking, asset management and trust group listed on the Luxembourg stock exchange. From 2001 to 2007 Mr. Breen also served as a non-executive director and co-owner of Damon Hill Holdings Limited, a multi franchise motor dealer group. Mr. Breen also serves as a director of a Cayman Islands fund, Bristol Investment Fund, Limited. Mr. Breen is also a non-executive director and co-owner of Colorsport Images Limited, a sports photographic agency and library. Mr. Breen is a U.K. qualified solicitor/attorney who holds an Honors LL.B. degree in law from the University College of Wales, Aberystwyth and qualified as a solicitor of the Supreme Court of Judicature of England and Wales in 1988. Mr. Breen is a former member of the International Bar Association, British Association for Sport and the Law, Law Society of England and Wales, and Holborn Law Society.
Alan Urban – Chief Financial Officer
Mr. Urban has previously served as a member of the Board of Directors of the Company from June 2022 to May 2023; as Chief Financial Officer for SRAX, Inc. (OTC: SRAX), a financial technology company, from March 2023 to July 2023; as Chief Financial Officer for Creek Road Miners, Inc. (formerly OTC: CRKR), a cryptocurrency mining company, from November 2021 to March 2023; and as Chief Financial Officer and Secretary for Research Solutions, Inc. (NASDAQ: RSSS), a SaaS and content provider in the scientific, technical and medical information space, from October 2011 to October 2021. Earlier in his career, Mr. Urban served as Chief Financial Officer and Senior Vice President of Finance and Accounting for ReachLocal, Inc. (NASDAQ: RLOC), an internet marketing company; and as Vice President of Finance and Treasurer for Infotrieve, Inc., a content provider in the scientific, technical and medical information space. He has been a Certified Public Accountant (currently inactive) since 1998. Mr. Urban received a B.S. in Business, with a concentration in Accounting Theory and Practice, from California State University, Northridge.
Bruce Wendel – Vice Chairman of the Board
Mr. Wendel was appointed to our Board of Directors on November 11, 2020. From April 2018 to May 2019, Mr. Wendel served as the Chief Business Development Officer for Prometic Biotherapeutics, Inc., a pharmaceutical development company. Mr. Wendel also served as Chief Strategic Officer of Hepalink USA, the U.S. subsidiary of Shenzhen Hepalink Pharmaceutical Company from February 2012 to July 2022, and Chief Executive Officer of Scientific Protein Laboratories, LLC from December 2014 to June 2015. He also served as a director of ProMetic Life Sciences Inc. and Vice Chairman and Chief Executive Officer at Abraxis BioScience, LLC, where he oversaw the development and commercialization of Abraxane® and led the negotiations that culminated in the acquisition of the company by Celgene Corporation in 2010. He began his 14 years at Bristol-Myers Squibb as in-house counsel before shifting to global business and corporate development where he served in roles of increasing responsibility. Subsequently, he was VP of Business Development at IVAX Corporation, and at American Pharmaceutical Partners, Inc. Mr. Wendel earned a juris doctorate degree from Georgetown University Law School, and a B.S. from Cornell University.
| 59 |
Rajesh Shrotriya, M.D. - Director
Dr. Shrotriya was appointed to our Board of Directors on January 13, 2021. Prior to joining our company, Dr. Shrotriya served as Chairman of the Board and Chief Executive Officer of Spectrum Pharmaceuticals, Inc. from August 2002 and a director since June 2001. From September 2000 to April 2014, Dr. Shrotriya also served as President of Spectrum Pharmaceuticals, Inc. and from September 2000 to August 2002, Dr. Shrotriya also served as Chief Operating Officer of Spectrum. Prior to joining Spectrum, Dr. Shrotriya held the position of Executive Vice President and Chief Scientific Officer from November 1996 until August 2000, and as Senior Vice President and Special Assistant to the President from November 1996 until May 1997, for SuperGen, Inc., a publicly-held pharmaceutical company focused on drugs for life-threatening diseases, particularly cancer. From August 1994 to October 1996, Dr. Shrotriya held the positions of Vice President, Medical Affairs and Vice President, Chief Medical Officer of MGI Pharma, Inc., an oncology-focused biopharmaceutical company. Dr. Shrotriya spent 18 years at Bristol-Myers Squibb Company, an NYSE-listed pharmaceutical company, in a variety of positions, most recently as Executive Director, Worldwide CNS Clinical Research. Previously, Dr. Shrotriya held various positions at Hoechst Pharmaceuticals, most recently as Medical Advisor. Dr. Shrotriya was an attending physician and held a courtesy appointment at St. Joseph Hospital in Stamford, Connecticut. In addition, he received a certificate for Advanced Biomedical Research Management from Harvard University. Dr. Shrotriya received an M.D. from Grant Medical College, Bombay, India, in 1974; a D.T.C.D. (Post Graduate Diploma in Chest Diseases) from Delhi University, V.P. Chest Institute, Delhi, India, in 1971; an M.B.B.S. (Bachelor of Medicine and Bachelor of Surgery — equivalent to an M.D. in the U.S.) from the Armed Forces Medical College, Poona, India, in 1967; and a B.S. in Chemistry from Agra University, Aligarh, India, in 1962. Currently, Dr. Shrotriya is on the Board of Trustees at the UNLV Foundation. Dr. Shrotriya is also a member of the Executive Committee, Audit Committee, and Development Committee of the University, and has been called to serve on the Recruitment Committee for the Dean of the Medical School.
Charles J. Casamento - Director
Mr. Casamento was appointed to our Board of Directors on May 1, 2023. Mr. Casamento is currently executive director and principal of The Sage Group, a healthcare advisory group specializing in mergers, acquisitions, and partnerships between biotechnology companies and pharmaceutical companies, since 2007. He was the president and CEO of Osteologix, Inc., a public biopharmaceutical company developing products for treating osteoporosis, from 2004 through 2007. Mr. Casamento was founder of, and from 1999 through 2004, served as chairman of the board, president and CEO, of Questcor Pharmaceuticals, Inc. which was subsequently acquired by Mallinckrodt Pharmaceuticals. Mr. Casamento formerly served as RiboGene, Inc.’s president, CEO and chairman of the board from 1993 through 1999 until it merged with Cypros Pharmaceutical Corp to form Questcor Pharmaceuticals, Inc. He was co-founder, president and CEO of Interneuron Pharmaceuticals, Inc. (Indevus), a biopharmaceutical company, from 1989 until 1993. Indevus was eventually acquired by Endo Pharmaceuticals. Mr. Casamento has also held senior management positions at Genzyme Corporation, where he was senior vice president, pharmaceuticals and biochemicals; American Hospital Supply, where he was vice president of business development and strategic planning for the Critical Care Division; Johnson & Johnson, Hoffmann-LaRoche, Inc. and Sandoz Inc. (now Novartis). Mr. Casamento also serves on the board of directors of the following Nasdaq listed companies: Eton Pharmaceuticals, Inc., PaxMedica, Inc. and Relmada Therapeutics, Inc. During his career he has served on the boards of fourteen Biotech/Pharma companies and has also been a director and vice chairman of The Catholic Medical Missions Board, a large not-for-profit organization providing health care services to third world countries. He has served as a guest lecturer at Fordham University and is on the Science Council of Fordham University. He holds a bachelor’s degree in Pharmacy from Fordham University and an MBA from Iona University and was originally licensed to practice pharmacy in the states of New York and New Jersey.
Term of Office
Each director serves until our next annual meeting or until his or her successor is duly elected and qualified. Each executive officer is elected by our board of directors and serves at its discretion.
Delinquent Section 16(a) Reports
Section 16(a) of the Exchange Act requires our officers, directors, and persons who own more than ten percent of a registered class of our equity securities to file reports of ownership and changes in ownership with the SEC and to furnish the Company with copies of all Section 16(a) forms they file. Our review of copies of the Section 16(a) reports filed to report transactions occurring during the fiscal year ended December 31, 2024 indicates that all filing requirements applicable to our officers, directors, and greater than ten percent beneficial owners were complied with.
| 60 |
Audit Committee and Audit Committee Financial Expert
Our Audit Committee currently consists of Mr. Casamento (Chairman), Dr. Shrotriya and Mr. Wendel. Our Board of Directors has determined that all current and prospective members of our Audit Committee are independent under the Nasdaq listing rules and Rule 10A-3(b)(1) of the Exchange Act. Our Board of Directors has determined that Mr. Casamento is an audit committee financial expert, as defined in Item 407(d)(5) of Regulation S-K, and that each member of our Audit Committee is able to read and understand fundamental financial statements and has substantial business experience that results in such member’s financial sophistication. Accordingly, our Board of Directors believes that each member of our Audit Committee has sufficient knowledge and experience necessary to fulfill such member’s duties and obligations on our Audit Committee.
Code of Conduct and Ethics
We have adopted a written code of conduct and ethics that applies to our directors, officers and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions. A current copy of the code is posted on the corporate governance section of our website, which is located at www.gtbiopharma.com. If we make any substantive amendments to, or grant any waivers from, the code of conduct and ethics for any officer or director, we will disclose the nature of such amendment or waiver on our website or in a current report on Form 8-K.
ITEM 11. EXECUTIVE COMPENSATION
Compensation of Executive Officers
The following table summarizes all compensation for the last two fiscal years awarded to, earned by, or paid to our Chief Executive Officer (principal executive officer) and our two most highly compensated executive officers other than our CEO who were either serving as executive officers at the end of our last completed fiscal year or for whom disclosure would have been provided but for the fact that the individual was not serving as an executive officer at the end of our last completed fiscal year, whose total compensation exceeded $100,000 during such fiscal year ends.
| Name and Principal Position | Year | Salary
($) | Bonus
($) | Stock
Awards (1) ($) | Option
Awards (2) ($) | All
Other Compensation ($) | Total
($) | |||||||||||||||||||||
| Michael Breen | 2024 | 556,200 | 417,150 | – | – | 213,622 | (3) | 1,186,972 | ||||||||||||||||||||
| Chairman, Interim Chief Executive Officer, and President | 2023 | 556,200 | 312,863 | 57,600 | (6) | 425,000 | (7) | 174,171 | (3) | 1,525,834 | ||||||||||||||||||
| Alan Urban | 2024 | 218,750 | 87,500 | – | 40,681 | (8) | 30,851 | (4) | 377,782 | |||||||||||||||||||
| Chief Financial Officer | 2023 | – | – | – | – | – | – | |||||||||||||||||||||
| Manu Ohri | 2024 | 203,540 | – | – | – | 104,264 | (5) | 307,804 | ||||||||||||||||||||
| Former Chief Financial Officer | 2023 | 432,000 | 172,800 | 57,600 | (9) | 425,000 | (10) | 42,271 | (5) | 1,129,671 | ||||||||||||||||||
| (1) | The amounts in this column represent the aggregate grant date fair value of restricted stock awards, determined in accordance with Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 718. We determine the grant date fair value of the awards by multiplying the number of units granted by the closing market price of one share of our common stock on the award grant date. These amounts do not reflect the actual economic value that will be realized by the named executive officer upon the vesting or the sale of the common stock awards. |
| (2) | This column represents option awards computed in accordance with FASB ASC Topic 718, excluding the effect of estimated forfeitures related to service-based vesting conditions. For additional information on the valuation assumptions with respect to the option grants, refer to Note 1 of our financial statements in the Annual Report. These amounts do not correspond to the actual value that will be recognized by the named executives from these awards. |
| (3) | Other compensation for 2024 includes $131,374 in paid time off payout, $60,000 in medical insurance, life insurance and long-term disability insurance premiums, and $22,248 in employer pension contributions. Other compensation for 2023 includes $128,833 in medical insurance, life insurance and long-term disability insurance premiums, and $45,338 in employer pension contributions. |
| (4) | Other compensation for 2024 includes $22,453 in medical insurance premiums, and $8,398 employer 401(k) contributions. |
| 61 |
| (5) | Other compensation for 2024 includes $78,300 in paid time off payout, and $25,964 in medical insurance premiums, life insurance and long-term disability insurance premiums, and medical expense reimbursements. Other compensation for 2023 includes $42,271 in medical insurance, life insurance and long-term disability insurance premiums, medical expense reimbursements and employer 401(k) contributions. |
| (6) | Represents the aggregate grant date fair value 6,666 shares of our common stock issued on August 11, 2023 at a closing market price of $8.70 per share as compensation for service as our Interim Chief Executive Officer. |
| (7) | Represents the aggregate grant date fair value of options to purchase 16,666 shares of common stock issued on January 27, 2023, with 1/12th of the shares vesting on the monthly anniversary of January 1, 2023 as compensation for service on our Board of Directors. |
| (8) | Represents the aggregate grant date fair value of options to purchase 23,335 shares of common stock issued on October 17, 2024, with 1/36th of the shares vesting on the monthly anniversary of June 3, 2024 until fully vested, subject to acceleration |
| (9) | Represents the aggregate grant date fair value 6,666 shares of our common stock issued on August 11, 2023 at a closing market price of $8.70 per share. |
| (10) | Represents the aggregate grant date fair value of options to purchase 16,666 shares of common stock issued on January 27, 2023, with 1/12th of the shares vesting on the monthly anniversary of January 1, 2023 |
Employment Agreements
The Company is party to employment agreements with both Michael Breen and Alan Urban, each of which are described below. The Company does not currently have employment agreements with any of its other officers and directors.
Michael Breen, Chairman, Interim Chief Executive Officer, and President
On December 31, 2021, the Company entered into a one-year, annually renewable executive services agreement with Mr. Breen, effective November 8, 2021, and amended on June 17, 2022 and February 20, 2023. Under the terms of the amended executive services agreement, Mr. Breen will receive an annual base salary of $556,200, reimbursement for executive life insurance premiums, a pension contribution equal to 4% of Mr. Breen’s gross annual salary, and right to receive any benefit and participate in any benefit plan generally available to the most senior level of employees of the Company. Mr. Breen is eligible to participate in our performance bonus plan or as otherwise determined by our Compensation Committee, with a target annual bonus of 75% of his annual base salary with a minimum guaranteed performance bonus of 25% of base salary.
Upon the termination of Mr. Breen’s services for any reason, Mr. Breen will receive his accrued but unpaid salary and vacation pay through the date of termination and any other benefits accrued to him under any benefit plans outstanding at such time, and the reimbursement of documented, unreimbursed expenses incurred prior to such date. Upon our termination of Mr. Breen’s services without cause (as defined in the his services agreement) or upon Mr. Breen’s termination of his employment for good reason (as defined in his services agreement) prior to the end of the term of his services agreement, Mr. Breen shall also receive (i) a lump sum payment equal to the greater of the amount of his annual base salary (at the then-current rate) that he would have earned through the end of the term of the agreement, and 50% of his annual base salary, plus (ii) a lump sum payment equal to the greater of the bonus paid or payable to Mr. Breen for the immediately preceding year, and the target bonus under our performance bonus plan, if any, in effect during the immediately preceding year, plus (iii) monthly reimbursement for the cost of medical, life and disability insurance coverage at a level equivalent to that provided by our company for a period of the earlier of (a) one year and (b) the time Mr. Breen begins alternative services wherein said insurance coverage is available and offered to Mr. Breen. Mr. Breen will also be designated for election to our Board of Directors during the term of his services agreement.
Alan Urban, Chief Financial Officer
On June 7, 2024, the Company entered into an Employment Agreement with Mr. Urban (the “Employment Agreement”). The Employment Agreement is effective from June 3, 2024 (the “Effective Date”) and shall continue for a period of one year. The Employment Agreement shall automatically renew for successive one-year periods unless and until either party provides sixty (60) days’ advance written notice prior to applicable renewal term. Pursuant to the Employment Agreement, Mr. Urban will receive an annual base salary of $375,000 and is eligible to earn an annual discretionary bonus of up to 40% of his annual base salary each calendar year during the term, subject to the achievement of applicable Company and individual performance goals, as determined in the Company’s sole discretion. Mr. Urban is eligible to receive a stock award of the Registrant’s Common Stock following the three months after the Effective Date. The Employment Agreement further provides that Mr. Urban will be eligible to receive any benefit and participate in any benefit plan generally available to employees of the Company.
| 62 |
The Company may terminate the Employment Agreement without Cause (as such term is defined in the Employment Agreement) at any time and Mr. Urban may terminate his employment for Good Reason (as such term is defined in the Employment Agreement) at any time. Upon a termination of Mr. Urban’s employment by the Company without Cause or by Mr. Urban for Good Reason, Mr. Urban will be entitled to receive (i) for a termination or resignation that occurs during the first six months following the Effective Date, a cash severance equal to two (2) months of Mr. Urban’s then current Annual Base Salary (as such term is defined in the Employment Agreement) or (ii) for a termination or resignation that occurs any time thereafter, a cash severance equal to five (5) months of Mr. Urban’s then current Annual Base Salary, in either case less deductions and withholding required by law, payable in a lump sum within seventy (70) days of the termination of employment (or such shorter period as may allow the severance payment to be exempt from Code Section 409A). Upon a termination of Mr. Urban’s employment by the Company for Cause or by Mr. Urban without Good Reason, Mr. Urban will be entitled to the Accrued Amounts (as such term is defined in the Employment Agreement). The Employment Agreement also contains certain non-disclosure covenants that apply during his employment and thereafter.
Outstanding Equity Awards at Fiscal Year End
The following table sets forth information regarding unexercised stock options for each named executive officer as of December 31, 2024.
| Name | Number of securities underlying unexercised options exercisable (#) | Number of securities underlying unexercised options unexercisable (#) | Option exercise price ($) | Option expiration date | ||||||||||
| Michael Breen | 1,666 | (1) | — | 74.40 | 7/14/2032 | |||||||||
| 16,666 | (1) | — | 25.50 | 1/27/2033 | ||||||||||
| Alan Urban | 4,537 | (2) | 18,798 | (2) | 2.11 | 10/17/2034 | ||||||||
| (1) | Consists of options granted to Mr. Breen as compensation for service on our Board of Directors. |
| (2) | Consists of options granted to Mr. Urban that vest as follows: 1/36th of the shares vest on the monthly anniversary of June 3, 2024 until fully vested, subject to acceleration. |
Compensation of Directors
The following table presents information regarding compensation awarded or paid to our non-employee directors for our fiscal year ended December 31, 2024 for the services rendered by them to the Company in all capacities.
| Name | Fees earned or paid in cash ($) | Stock awards ($) | Option awards ($) | Total ($) | ||||||||||||
| Rajesh Shrotriya, M.D. | 81,000 | — | — | 81,000 | ||||||||||||
| Bruce Wendel | 81,000 | — | — | 81,000 | ||||||||||||
| Charles J. Casamento | 65,000 | — | — | 65,000 | ||||||||||||
During fiscal 2024, our non-employee directors received annual cash compensation in the amount of $50,000 for service on our Board of Directors, and annual compensation of $5,000 per committee for service on our Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee. Non-employee members of the Special Committee of our Board of Directors also received a monthly fee of $8,000 for service on the Special committee for the months of January and February 2024.
Indemnification of Directors and Executive Officers and Limitation of Liability
We have entered into indemnification agreements with each of our current directors and certain key employees. The indemnification agreements, our restated certificate of incorporation and Amended Restated Bylaws require us to indemnify our current and former directors and officers to the fullest extent permitted by Delaware law.
| 63 |
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information, as of February 19, 2025, with respect to the holdings of (1) each person who is the beneficial owner of more than five percent of our common stock, (2) each of our directors, (3) each named executive officer, and (4) all of our directors and executive officers as a group.
Beneficial ownership of our common stock is determined in accordance with the rules of the Securities and Exchange Commission and includes any shares of common stock over which a person exercises sole or shared voting or investment powers, or of which a person has a right to acquire ownership at any time within 60 days of February 19, 2025. Except as otherwise indicated, and subject to applicable community property laws, the persons named in this table have sole voting and investment power with respect to all shares of common stock held by them. The address of each director and officer is 315 Montgomery Street, 10th Floor, San Francisco, California 94104. Applicable percentage ownership in the following table is based on 2,234,328 shares of common stock outstanding as of February 19, 2025 plus, for each person, any securities that person has the right to acquire within 60 days of February 19, 2025.
| Name of Beneficial Owner | Number of Shares Beneficially Owned | Percentage of Shares Outstanding | ||||||
| Five Percent or Greater Stockholders: | ||||||||
| Cytovance Biologics, Inc. (1) | 219,457 | 9.8 | % | |||||
| Robert A. Marzilli (2) | 200,000 | 9.0 | % | |||||
| Bristol Investment Fund, Ltd. (3) | 190,000 | 7.8 | % | |||||
| Executive Officers and Directors: | ||||||||
| Michael Breen (4) | 46,872 | 2.1 | % | |||||
| Alan Urban (5) | 4,537 | 0.2 | % | |||||
| Bruce Wendel (6) | 31,753 | 1.4 | % | |||||
| Rajesh Shrotriya, M.D. (7) | 32,047 | 1.4 | % | |||||
| Charles J. Casamento (8) | 16,666 | 0.7 | % | |||||
| Directors and officers as a group (5 persons) (9) | 131,875 | 5.7 | % | |||||
| (1) | The address for Cytovance Biologics, Inc. is 800 Research Parkway, Suite 200 Oklahoma City, OK 73104. |
| (2) | The address for Robert A. Marzilli is 457 Sunset Beach Rd., Richmond Hill, Ontario, L4E 3J3, Canada. This information is based on information known to the company through a non-objecting beneficial ownership report (the “NOBO Report”) as of December 31, 2024. Mr. Marzilli has not provided or verified the information appearing on the NOBO Report, and so this information may not be accurate for a number of reasons, including, but not limited to, if Mr. Marzilli has divested such ownership through private contractual or other means not reflected in the NOBO Report, or is the beneficial owner of other shares not disclosed in the NOBO Report. |
| (3) | The address for Bristol Investment Fund, Ltd. is Citco Trustees (Cayman) Limited, 89 Nexus Way, Camana Bay, PO Box 311063, Grand Cayman KY1-1205, Cayman Islands. Includes shares underlying warrants to purchase 190,000 shares of common stock at $4.35 per share. |
| (4) | Includes shares underlying options to purchase 1,666 shares of common stock at $74.40 per share, and 16,666 shares of common stock at $25.50 per share. |
| (5) | Includes shares underlying options to purchase 4,537 shares of common stock at $2.11 per share. |
| (6) | Consists of shares underlying options to purchase 3,332 shares of common stock at $74.40 per share, and 16,666 shares of common stock at $25.50 per share. |
| (7) | Consists of shares underlying options to purchase 15,381 shares of common stock at $74.40 per share, and 16,666 shares of common stock at $25.50 per share. |
| (8) | Includes shares underlying options to purchase 16,666 shares of common stock at $10.50 per share. |
| (9) | Includes shares underlying options to purchase 91,580 shares of common stock. |
Equity Compensation Plan Information
In 2014 we established the 2014 Stock Incentive Plan (the “2014 Plan”) and in April 2022 we established the 2022 Omnibus Incentive Plan, collectively (the “Plans”). The Plans were approved by our Board of Directors and stockholders. The purpose of the Plans is to grant stock and options to purchase our common stock, and other incentive awards, to our employees, directors and key consultants. The maximum number of shares of common stock that may be issued pursuant to awards granted under the 2022 Plan is 166,667. Following adoption of the 2022 Plan by our stockholders we only grant incentive awards under the 2022 Plan. The shares of our common stock underlying cancelled and forfeited awards issued under the 2022 Plan may again become available for grant under the 2022 Plan. As of December 31, 2024, there were no shares available for grant under the 2014 Plan. All outstanding incentive stock award grants prior to the adoption of the 2022 Plan were made under the 2014 Plan, and all incentive stock award grants after the adoption of the 2022 Plan have been and will be made under the 2022 Plan. The following table provides information as of December 31, 2024 with respect to the Plans.
| 64 |
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted average exercise price of outstanding options, warrants and rights (1) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by stockholders (2014 Plan) | — | — | ||||||||||
| Equity compensation plans approved by stockholders (2022 Plan) | 150,535 | $ | 32.69 | 16,132 | ||||||||
| Total | 150,535 | 16,132 | ||||||||||
| (1) | The weighted average exercise price excludes restricted stock awards, which have no exercise price. |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Officers, Directors and greater than 5% stockholders
Other than listed below, since January 1, 2023, there has not been, nor is there currently proposed, any transaction or series of similar transactions to which we were or will be a party:
| ● | in which the amount involved exceeds the lesser of $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years; and |
| ● | in which any director, executive officer, stockholder who beneficially owns more than 5% of our common stock or any member of their immediate family had or will have a direct or indirect material interest. |
Cytovance
In October 2020, the Company entered into a Master Services Agreement with Cytovance Biologics, Inc. (“Cytovance”), to perform biologic development and manufacturing services, and to produce and test compounds used in the Company’s potential product candidates. The Company subsequently executed numerous Statements of Work (“SOWs”) for the research and development of products for use in clinical trials. On August 24, 2022, the Company entered into a Settlement and Investment Agreement with Cytovance that amended existing SOWs and allowed for future invoices to be settled in in a combination of cash and issuance of the Company’s common stock. The Agreement also set Cytovance’s beneficial ownership limitation at 4.9% of the issued and outstanding shares of the Company’s common stock. On April 25, 2024, the Company entered into an Amendment to the Settlement and Investment Agreement with Cytovance that increased Cytovance’s beneficial ownership limitation to 9.9% of the issued and outstanding shares of the Company’s common stock. On June 30, 2024, Cytovance became a related party as their beneficial ownership exceeded 5% of the issued and outstanding shares of the Company’s common stock.
During the years December 31, 2024 and 2023, the Company recognized research and development expenses of $2,335,000 and $4,584,000, respectively and made cash payments amounting to $3,857,000 and $2,213,000, respectively to Cytovance. In addition, the Company issued 127,597 and 57,437 shares of common stock to Cytovance to settle accounts payable valued at approximately $810,000 and $1,120,000, respectively. As of December 31, 2024 the Company’s commitments in relation to unbilled and unaccrued SOWs and any related Change Orders from Cytovance for services that have not yet been rendered as of December 31, 2024, amounted to approximately $1.1 million. As of December 31, 2024 and 2023, accounts payable to Cytovance amounted to $1,183,000 and $3,515,000, respectively.
| 65 |
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES
Summary of Principal Accounting Fees for Professional Services Rendered
Our independent registered public accounting firm is Weinberg & Company, P.A. 1925 Century Park E., Suite1120, Los Angeles, CA 90067. PCAOB Auditor ID: 572. The following table presents the aggregate fees for professional audit services and other services rendered in the fiscal years ended December 31, 2024 and 2023.
The following table presents the aggregate fees for professional audit services and other services rendered by Weinberg in the fiscal years ended December 31, 2024 and 2023.
| Year Ended December 31, 2024 | Year Ended December 31, 2023 | |||||||
| Audit Fees | $ | 199,925 | $ | 185,828 | ||||
| Audit Related Fees | — | — | ||||||
| Tax Fees | 28,489 | 39,907 | ||||||
| All Other Fees | — | — | ||||||
| Total | $ | 228,414 | $ | 225,735 | ||||
Audit Fees consist of amounts billed for professional services rendered for the audit of our annual financial statements included in our Annual Reports on Form 10-K, and reviews of our interim financial statements included in our Quarterly Reports on Form 10-Q.
Audit-Related Fees consist of fees billed for professional services that are reasonably related to the performance of the audit or review of our financial statements but are not reported under “Audit Fees.”
Tax Fees consist of fees for professional services for tax compliance activities, including the preparation of federal and state tax returns and related compliance matters.
All Other Fees consists of amounts billed for services other than those noted above.
The audit committee of our board of directors has considered whether the provision of the services described above for the fiscal years ended December 31, 2024 and 2023, is compatible with maintaining the auditor’s independence.
All audit and non-audit services that may be provided by our principal accountant to us shall require pre-approval by the audit committee of our board of directors. Further, our auditor shall not provide those services to us specifically prohibited by the SEC, including bookkeeping or other services related to the accounting records or financial statements of the audit client; financial information systems design and implementation; appraisal or valuation services, fairness opinion, or contribution-in-kind reports; actuarial services; internal audit outsourcing services; management functions; human resources; broker-dealer, investment adviser, or investment banking services; legal services and expert services unrelated to the audit; and any other service that the Public Company Accounting Oversight Board determines, by regulation, is impermissible.
| 66 |
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements.
The financial statements of GT Biopharma, Inc. and the independent registered public accounting firm’s report dated February 21, 2025, are incorporated by reference to Item 8 of this report.
(a)(2) and (c) Financial Statement Schedules
Not required.
(a)(3) and (b) Exhibits
EXHIBIT INDEX
| 67 |
| 68 |
| 69 |
++ Indicates management contract or compensatory plan.
* This certification shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, or otherwise subject to the liability of that Section, nor shall it be deemed to be incorporated by reference into any filing under the Securities Act or the Exchange Act.
**The Registrant has omitted portions of this exhibit that are both not material and the type of information that the Registrant treats as private or confidential.
ITEM 16. FORM 10-K SUMMARY
Not applicable.
| 70 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
GT Biopharma, Inc. | ||
| Dated: February 21, 2025 | By: | /s/ Michael Breen |
Michael Breen, Executive Chairman of the Board and Interim Chief Executive Officer (Principal Executive Officer) | ||
GT Biopharma, Inc. | ||
| Dated: February 21, 2025 | By: | /s/ Alan Urban |
| Alan Urban, Chief Financial Officer | ||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Michael Breen and Alan Urban, and each of them, as his true and lawful attorneys-in-fact and agents, with full power of substitution for him, and in his name in any and all capacities, to sign any and all amendments to this Annual Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, and any of them or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Name | Position | Date | ||
|
||||
| /s/ Michael Breen | Executive Chairman of the Board and | February 21, 2025 | ||
| Michael Breen | Interim Chief Executive Officer (Principal Executive Officer) | |||
|
||||
| /s/ Alan Urban | Chief Financial Officer | February 21, 2025 | ||
| Alan Urban | (Principal Financial and Accounting Officer) | |||
|
||||
| /s/ Bruce Wendel | Vice Chairman of the Board | February 21, 2025 | ||
| Bruce Wendel | ||||
|
||||
| /s/ Rajesh Shrotriya | Director | February 21, 2025 | ||
| Rajesh Shrotriya, M.D. | ||||
|
||||
| /s/ Charles Casamento | Director | February 21, 2025 | ||
| Charles Casamento |
| 71 |