

Forward Looking Statements This presentation and the information contained herein does not constitute or form part of, and should not be construed as, an offer or invitation to sell or subscribe for, or a solicitation of any offer or invitation to acquire, dispose of or subscribe for, securities of Oxis International, Inc., GT Biopharma, Inc., or Georgetown Translational Pharmaceuticals, Inc. (the “Company") in any country where such offer, invitation or subscription would be prohibited by law. The publication of this presentation in certain countries may violate applicable regulations.This presentation contains forward-looking statements that can be identified by such terminology such as “expects”, “potential”, “suggests”, “may”, or similar expressions. Such forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause the actual results to be materially different from any future results, performance or achievements expressed or implied by such statements. In particular, management’s expectations regarding future research and development results could be affected by, among other things, uncertainties relating to clinical trials and product development; unexpected regulatory delays or government regulation generally; the Company’s ability to obtain or maintain patent and other proprietary intellectual property protection; and competition in general. Forward-looking statements speak only as to the date they are made. The Company does not undertake to update forward-looking statements to reflect circumstances or events that occur after the date the forward looking statements are made.

Pending Merger Rationale Oxis International Inc. (OTCBB:OXIS)Novel immuno-oncology platforms with lead product candidate currently in Phase 2 clinical trialGeorgetown Translational Pharmaceuticals, Inc. (Private)505(b)(2)-focused CNS product candidate portfolioMore rapid development timelines anticipated to provide potential product approvals during more lengthy oncology timelinesAdds CEO with successful track record of developing drugs through FDA approvalCombined company expected to provide multiple opportunities for value creation in both near and long-term time horizons

Investment Highlights Diversified drug development pipeline across two therapeutic categories – oncology and CNSPipeline offers more balanced risk/reward profile with potential for steady news flowLead immuno-oncology product candidate, OXS-1550, a novel bispecific antibody drug conjugate, currently in Phase 2Lead CNS product candidate, PainBrake, anticipated to be NDA-ready in ~15-18 monthsExperienced management team with track record of success in drug development and successful exits
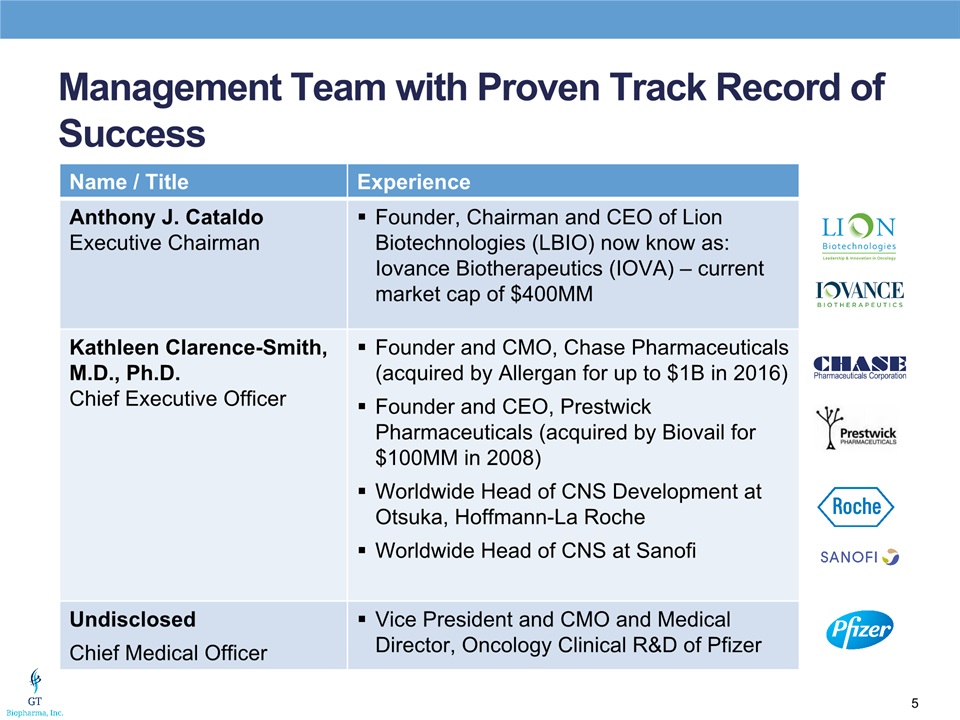
Management Team with Proven Track Record of Success Name / Title Experience Anthony J. CataldoExecutive Chairman Founder, Chairman and CEO of Lion Biotechnologies (LBIO) now know as: Iovance Biotherapeutics (IOVA) – current market cap of $400MM Kathleen Clarence-Smith, M.D., Ph.D.Chief Executive Officer Founder and CMO, Chase Pharmaceuticals (acquired by Allergan for up to $1B in 2016)Founder and CEO, Prestwick Pharmaceuticals (acquired by Biovail for $100MM in 2008)Worldwide Head of CNS Development at Otsuka, Hoffmann-La RocheWorldwide Head of CNS at Sanofi UndisclosedChief Medical Officer Vice President and CMO and Medical Director, Oncology Clinical R&D of Pfizer
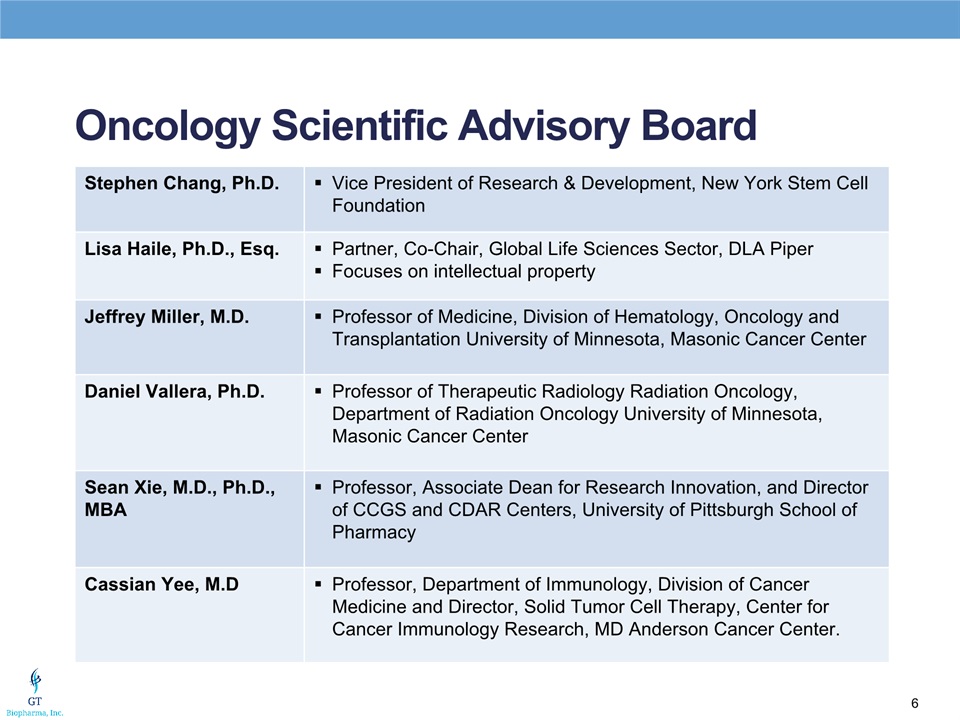
Oncology Scientific Advisory Board Stephen Chang, Ph.D. Vice President of Research & Development, New York Stem Cell Foundation Lisa Haile, Ph.D., Esq. Partner, Co-Chair, Global Life Sciences Sector, DLA PiperFocuses on intellectual property Jeffrey Miller, M.D. Professor of Medicine, Division of Hematology, Oncology and Transplantation University of Minnesota, Masonic Cancer Center Daniel Vallera, Ph.D. Professor of Therapeutic Radiology Radiation Oncology, Department of Radiation Oncology University of Minnesota, Masonic Cancer Center Sean Xie, M.D., Ph.D., MBA Professor, Associate Dean for Research Innovation, and Director of CCGS and CDAR Centers, University of Pittsburgh School of Pharmacy Cassian Yee, M.D Professor, Department of Immunology, Division of Cancer Medicine and Director, Solid Tumor Cell Therapy, Center for Cancer Immunology Research, MD Anderson Cancer Center.
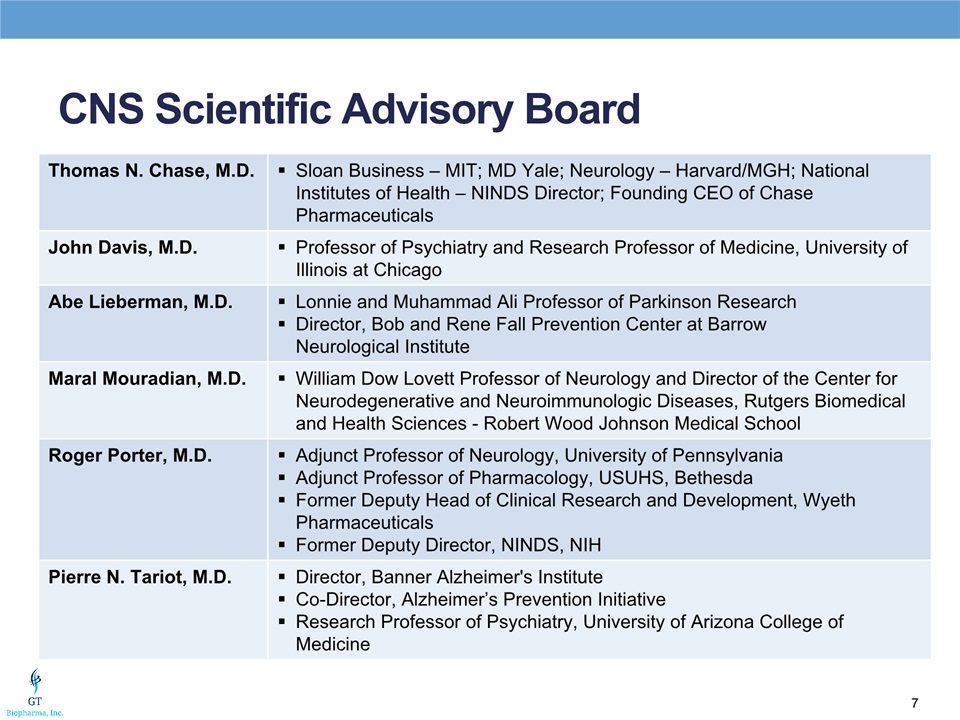
CNS Scientific Advisory Board Thomas N. Chase, M.D. Sloan Business – MIT; MD Yale; Neurology – Harvard/MGH; National Institutes of Health – NINDS Director; Founding CEO of Chase Pharmaceuticals John Davis, M.D. Professor of Psychiatry and Research Professor of Medicine, University of Illinois at Chicago Abe Lieberman, M.D. Lonnie and Muhammad Ali Professor of Parkinson ResearchDirector, Bob and Rene Fall Prevention Center at Barrow Neurological Institute Maral Mouradian, M.D. William Dow Lovett Professor of Neurology and Director of the Center for Neurodegenerative and Neuroimmunologic Diseases, Rutgers Biomedical and Health Sciences - Robert Wood Johnson Medical School Roger Porter, M.D. Adjunct Professor of Neurology, University of PennsylvaniaAdjunct Professor of Pharmacology, USUHS, BethesdaFormer Deputy Head of Clinical Research and Development, Wyeth PharmaceuticalsFormer Deputy Director, NINDS, NIH Pierre N. Tariot, M.D. Director, Banner Alzheimer's InstituteCo-Director, Alzheimer’s Prevention InitiativeResearch Professor of Psychiatry, University of Arizona College of Medicine
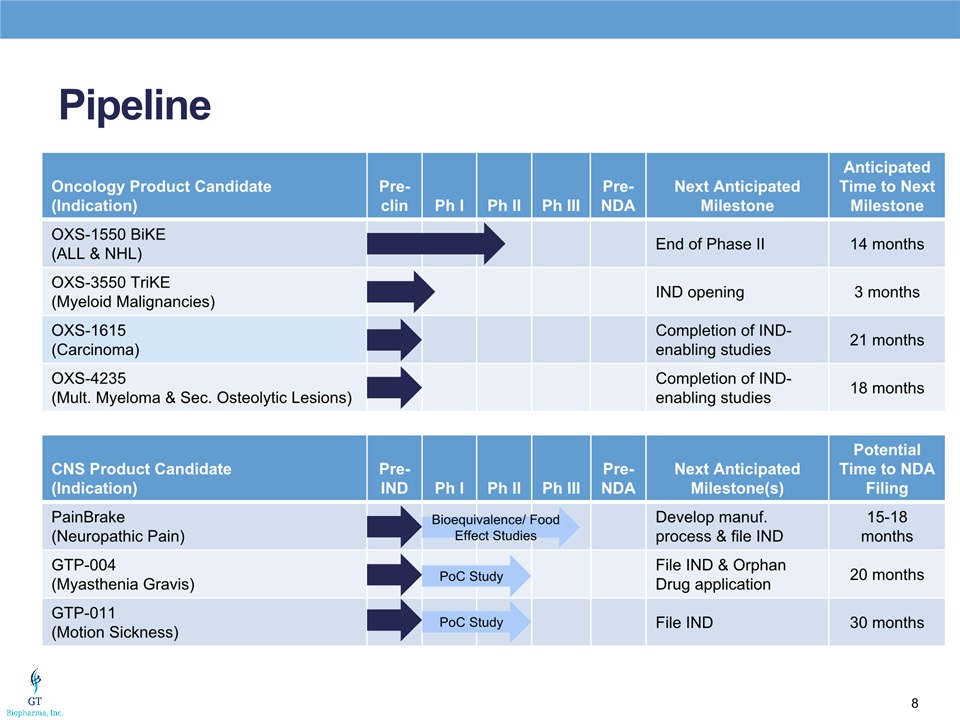
Pipeline Oncology Product Candidate(Indication) Pre-clin Ph I Ph II Ph III Pre-NDA Next Anticipated Milestone Anticipated Time to Next Milestone OXS-1550 BiKE(ALL & NHL) End of Phase II 14 months OXS-3550 TriKE(Myeloid Malignancies) IND opening 3 months OXS-1615(Carcinoma) Completion of IND-enabling studies 21 months OXS-4235(Mult. Myeloma & Sec. Osteolytic Lesions) Completion of IND-enabling studies 18 months CNS Product Candidate(Indication) Pre-IND Ph I Ph II Ph III Pre-NDA Next Anticipated Milestone(s) Potential Time to NDA Filing PainBrake(Neuropathic Pain) Develop manuf. process & file IND 15-18 months GTP-004(Myasthenia Gravis) File IND & Orphan Drug application 20 months GTP-011(Motion Sickness) File IND 30 months Bioequivalence/ Food Effect Studies PoC Study PoC Study

Oncology Portfolio
Novel Immuno-Oncology Technology Platforms Antibody-Drug Conjugate (ADC) Therapeutic PlatformscFvs targeting CD19, CD22, CD33, CD133, TAG72Full-length human MAbs targeting ICAM, FZD7, CD38Payloads: Diphtheria Toxin, OXS-4235, Pseudomonas ExotoxinAntibody Directed Cell-Mediated Cytotoxic (ADCC) Therapeutic PlatformNK Cell Engagers (Core: CD16 scFv/IL15)T-Cells Engagers (Core: CD3 scFv /TAG-72 w/wo cytokines or immune checkpoint inhibitors)
Currently Approved ADC and ADCC Therapies Blincyto® (blinatumomab) from Amgen is a first-in-class FDA approved bispecific CD19-directed CD3 T-cell engager antibody directed cell-mediated cytotoxic therapeutic (ADCC). Blinctyo is approved for the treatment of childhood acute lymphoblastic leukemia. ADCETRIS® (brentuximab vedotin) from Seattle Genetics is a first-in-class FDA approved antibody-drug conjugate (ADC) targeting CD30+ cancer cells. Adcetris is approved for the treatment of certain lymphomas.

OXS-1550 BiKEDual targeting delivering diphtheria toxin payload Bispecific killer engager (BiKE) ADC targeting CD19+ and CD22+ cancers with de-immunized diphtheria toxin payload Indication: CD19+ and CD22+ B-cell malignancies:Acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma (NHL)Broader therapeutic activity than competitive products resulting from bispecific scFv ADC design able to target cancer cells expressing CD19 or CD22 or both receptors Potent de-immunized diphtheria toxin payload disrupts cancer cell protein synthesis via inhibition of elongation factor-2 (EF-2)Phase I dose-escalation completed - DLT between 40 and 60 μg/kg – Durable response in 2 patientsFirst patient enrolled in Phase II clinical trial in April 2017Licensed from University of Minnesota
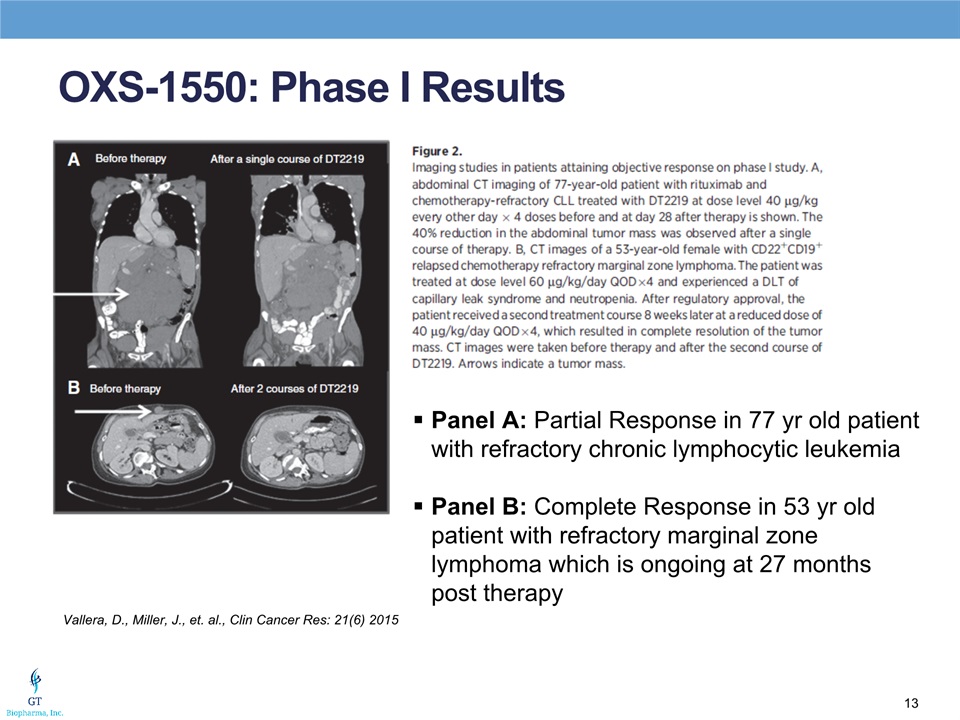
OXS-1550: Phase I Results Vallera, D., Miller, J., et. al., Clin Cancer Res: 21(6) 2015 Panel A: Partial Response in 77 yr old patient with refractory chronic lymphocytic leukemiaPanel B: Complete Response in 53 yr old patient with refractory marginal zone lymphoma which is ongoing at 27 months post therapy

OXS-1550: Phase II Trial Design Study Design Continuation Phase I dose/schedule finding component using MTD identified during previous Phase I study, but with a higher number of dosesTwo-stage Phase II extension component to confirm safety and make a preliminary determination of activity level by disease using the dose identified in Phase I Dosing / Disease Assessment Minimum of 1 cycle of OXS-1550I.V. infusion on days 1, 3, 5, 8 and days 15, 17,19, 22 of a 28-day treatment cycleDisease reassessment on day 29If patient has clinical benefit and no unacceptable side effects, they may receive up to 2 additional cycles until disease progression and/or unacceptable toxicityDose cohorts: 40, 60, 80 µg/kg/dose Enrollment Criteria Patients ≥12 years with relapsed or refractory CD19+ and/or CD22+ B-lineage leukemia/lymphomaPhase I: Standard 3+3 design requiring 6 to 12 patientsPhase II: Two stage design by disease – assigned to Arm 1(NHL) or Arm 2 (Leukemia) stage 1: enroll a total of 9 patients (including all treated at the MTD in Phase I and evaluable after 1st cycle of treatment) if 1 or more responds within the Arm, activate stage 2 enrolling an additional 8 patients to that Arm Clinical Site Masonic Cancer Center, University of Minnesota Principal Investigator Veronika Bachanova, M.D., Ph.D. Co-Principal Investigators Jeffery S. Miller, M.D.Michael Verneris, M.D.Aleksandr Lazaryan, M.D., M.P.H., Ph.D. IND Sponsor Daniel Vallera, Ph.D.
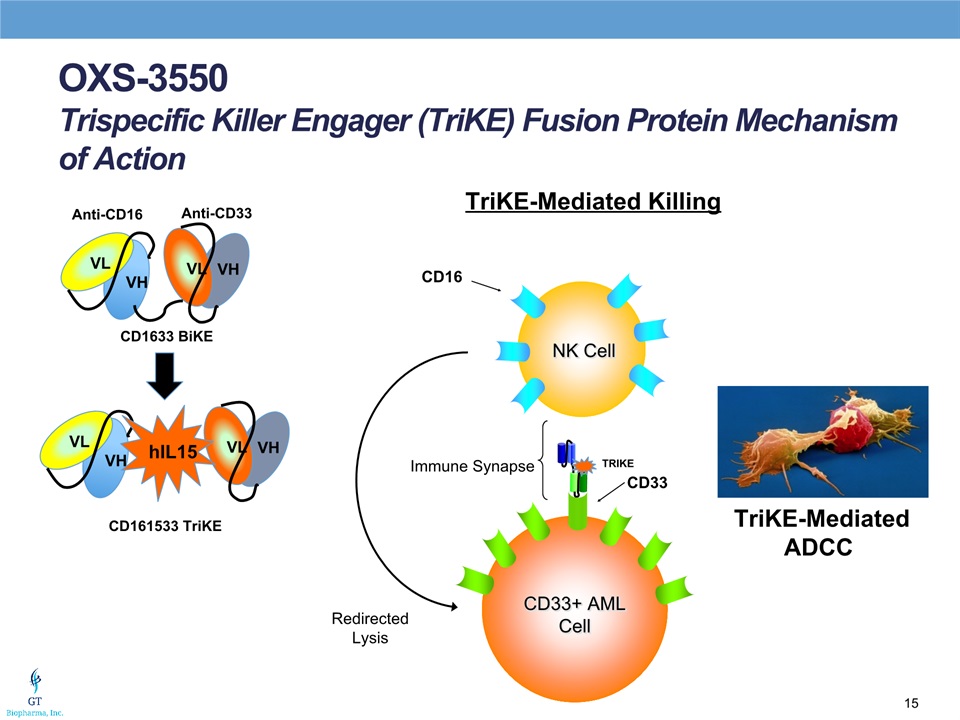
Anti-CD16 Anti-CD33 VL VH VL VH CD1633 BiKE CD161533 TriKE VL VH VL VH hIL15 NK Cell CD33+ AML Cell Immune Synapse CD16 CD33 Redirected Lysis TriKE-Mediated Killing TRIKE TriKE-MediatedADCC OXS-3550Trispecific Killer Engager (TriKE) Fusion Protein Mechanism of Action

OXS-3550TriKE for Myeloid Malignancies Trispecific ADCC NK Cell engager targeting CD16 receptor on NK cells and CD33 receptor on cancer cells Contains IL-15 as an NK cell activatorIndication CD33+ myeloid malignancies:Acute Myelogenous Leukemia (AML)Myelodysplastic syndromeUnique incorporation of IL-15 into design yielding a trispecific scFv ADCC constructTargets CD33+ myeloid malignanciesBinds to the CD16 receptors on NK cells with IL-15 activating the NK cell regardless of the presence of killer cell immunoglobulin-like receptor (KIR) ligands which inhibit NK cell activityPre-IND meeting held on April 4, 2017- IND filed June 8, 2017Phase I clinical trial expected to begin Q3 20174 days continuous infusion x 3 weeks cycle courseDose finding and extended Phase II componentPartnership agreement with Altor BioScience Corp.Dr. Patrick Song-Shiong serves as Chairman of AltorLicensed from the University of Minnesota
OXS-1615TetraKE for Carcinoma Tetraspecific ADC targeting CD16/IL-15/EpCAM/CD133 Targets EpCAM+ present on the majority of solid tumors and CD133+, an establishe cancer stem cell markerPotential indications: carcinomas including breast, prostate, pancreatic, head and neck, colon, kidney, liver, and lungLicensed from University of Minnesota

OXS-4235Starting development: Small molecule for multiple myeloma Small molecule P62-ZZ inhibitor targeting the treatment of multiple myeloma and associated osteolytic lesionsIn in vitro and in vivo models of multiple myeloma and osteoporosis, OXS-4235 demonstrated the ability to kill multiple myeloma cells, and decrease osteolytic lesions in bonePre-IND meeting anticipated for Q1 of 2018Licensed from Dr. Sean Xie of University Of Pittsburgh

CNS Portfolio
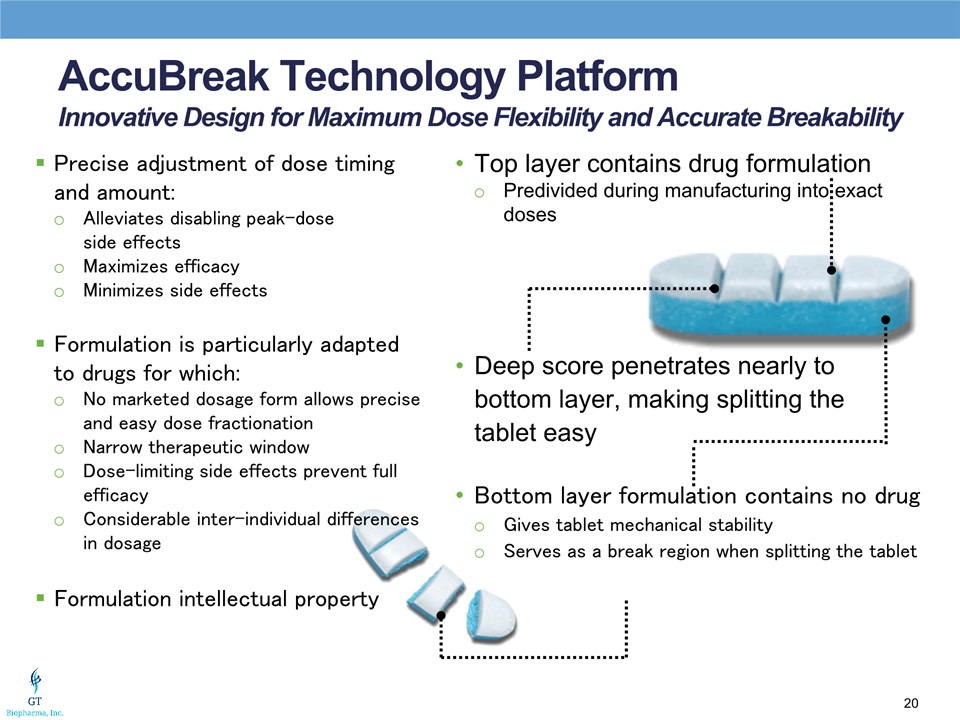
AccuBreak Technology PlatformInnovative Design for Maximum Dose Flexibility and Accurate Breakability Precise adjustment of dose timing and amount:Alleviates disabling peak-dose side effectsMaximizes efficacyMinimizes side effectsFormulation is particularly adapted to drugs for which:No marketed dosage form allows precise and easy dose fractionationNarrow therapeutic windowDose-limiting side effects prevent full efficacyConsiderable inter-individual differences in dosageFormulation intellectual property Top layer contains drug formulationPredivided during manufacturing into exact doses Bottom layer formulation contains no drug Gives tablet mechanical stabilityServes as a break region when splitting the tablet Deep score penetrates nearly to bottom layer, making splitting the tablet easy

AccuBreak Tablets Have Relatively Straightforward Path to NDA Approval For the NDA to be a 505(b)(2)Total tablet strength must remain the same as initial tabletPackage insert must remain the same (except for new tablet description)Only 2 Phase I studies in healthy volunteers (N=24 to 48) are expected to be needed for NDA filing + 3 commercial tablet batchesSpecial and expensive equipment is required for tablet manufacturing -Contracted with a US manufacturer who has the equipment

Chronic Neuropathic PainUrgent Need for Better Drugs – Prevalence Increasing with Aging Population Neuropathic pain reflects a chronic dysfunction of the nervous system Causes include diabetic neuropathy, postherpetic neuralgia, certain forms of chemotherapy, trauma, and multiple sclerosis ~16 MM Americans suffer from chronic neuropathic pain and the prevalence is increasing due to aging population(1)Current drugs provide a useful degree of pain relief in only about half the patientsOnly 1 in 4 patients experiences >50% pain relief Very few patients achieve complete relief of pain; ~30% of patients have no or very little relief Peak dose-limiting side effects (mainly sedation) cause patients to be under-dosed, thereby contributing to inadequate pain relief Yawn et. al., 2009
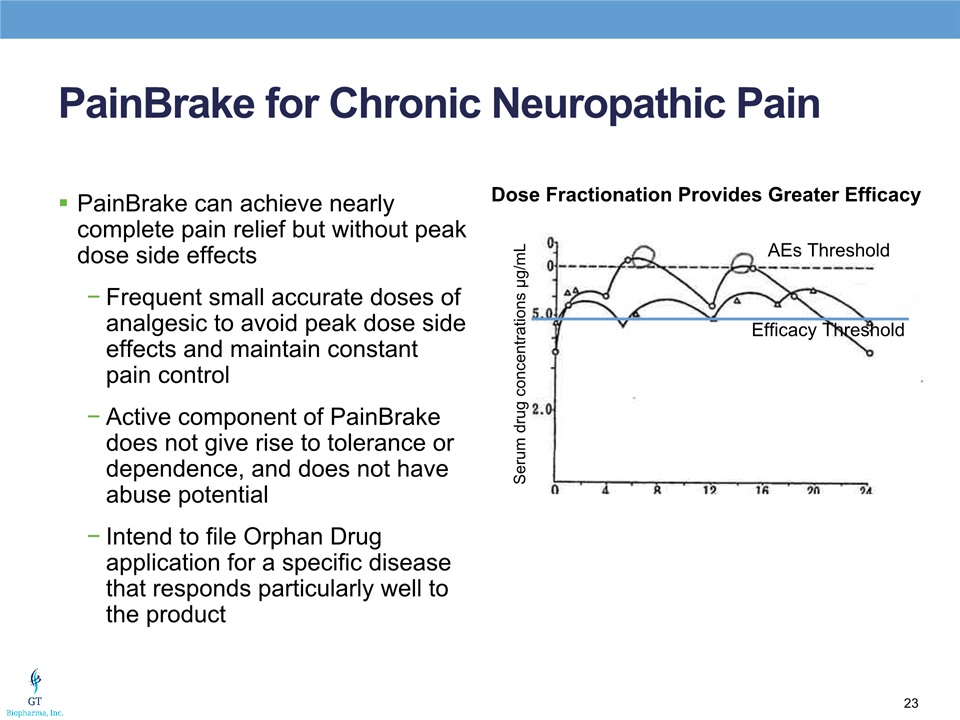
PainBrake for Chronic Neuropathic Pain PainBrake can achieve nearly complete pain relief but without peak dose side effectsFrequent small accurate doses of analgesic to avoid peak dose side effects and maintain constant pain controlActive component of PainBrake does not give rise to tolerance or dependence, and does not have abuse potentialIntend to file Orphan Drug application for a specific disease that responds particularly well to the product Dose Fractionation Provides Greater Efficacy AEs Threshold Efficacy Threshold Serum drug concentrations μg/mL
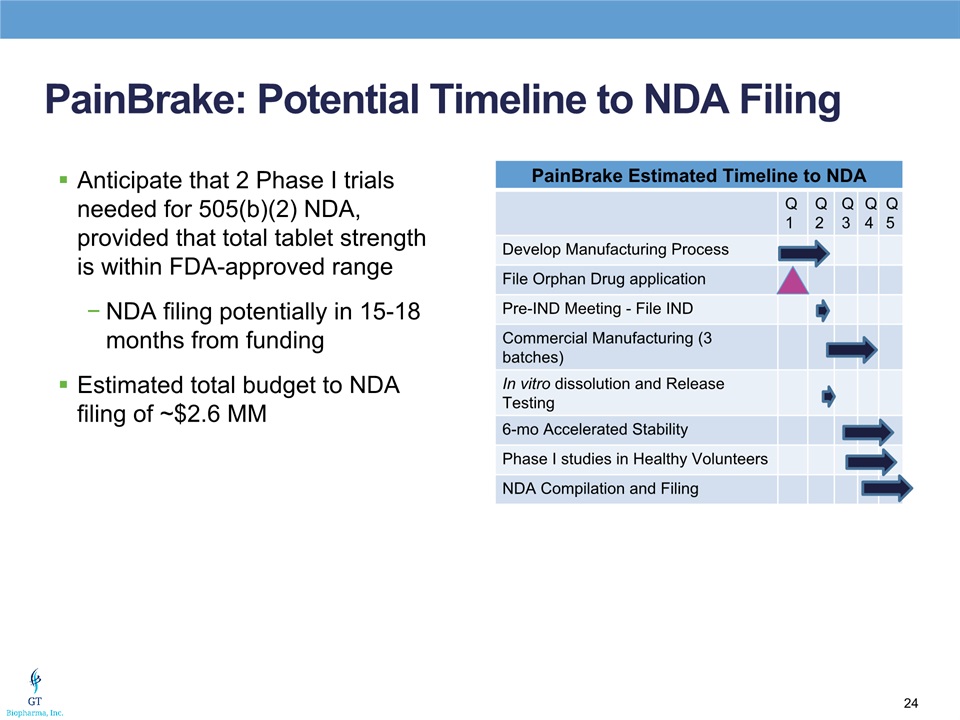
PainBrake: Potential Timeline to NDA Filing Anticipate that 2 Phase I trials needed for 505(b)(2) NDA, provided that total tablet strength is within FDA-approved rangeNDA filing potentially in 15-18 months from fundingEstimated total budget to NDA filing of ~$2.6 MM PainBrake Estimated Timeline to NDA Q1 Q2 Q3 Q4 Q5 Develop Manufacturing Process File Orphan Drug application Pre-IND Meeting - File IND Commercial Manufacturing (3 batches) In vitro dissolution and Release Testing 6-mo Accelerated Stability Phase I studies in Healthy Volunteers NDA Compilation and Filing

GTP-004 for Myasthenia GravisAddresses Medical Need for Safer and Better Tolerated Treatment Only one drug, Pyridostigmine, currently approved for this rare muscular diseaseApproved drug restores muscle strength to pre-disease state, but GI side effects (nausea, vomiting, diarrhea) are dose limitingDisease causes patients to be wheelchair bound~60,000 patients in the US1 GI adverse effects are an important source of discomfort, may lead to non-compliance or decrease the dose of efficacious treatment GTP-004 combines Pyridostigmine with an approved antagonist of the GI side effectsFixed dose combination of the two approved drugsOrphan Drug application filedPatents filed in early 2017 Source: 1Howard JF. Clinical Overview of MG. Myastenia Gravis Foundation of America Accessed at http://www.myasthenia.org/HealthProfessionals/ClinicalOverviewofMG.aspx on May 22, 2017
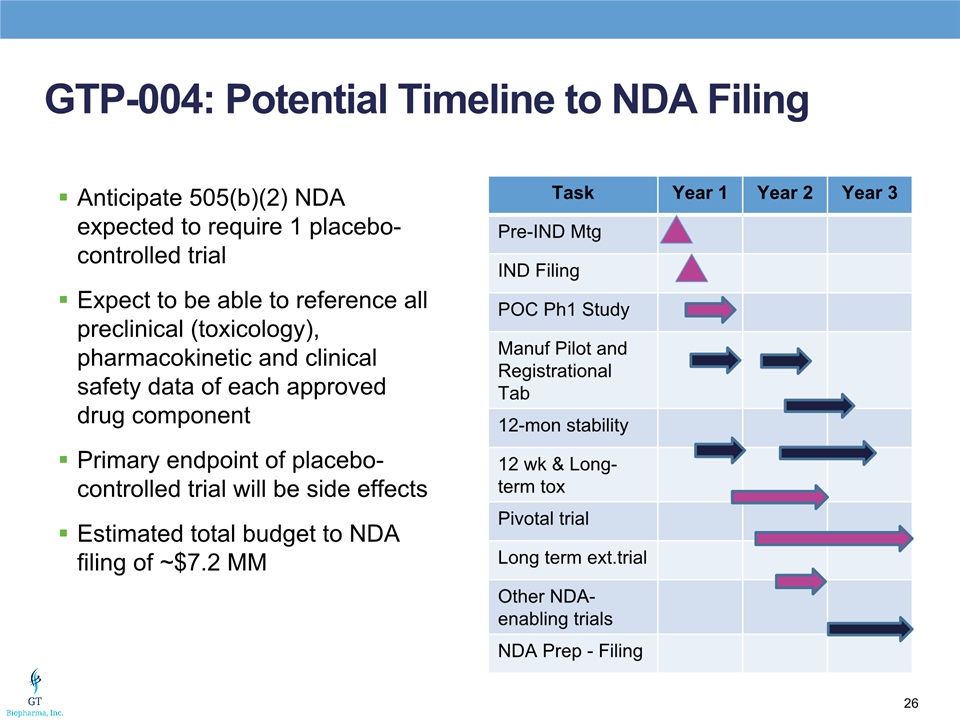
GTP-004: Potential Timeline to NDA Filing Anticipate 505(b)(2) NDA expected to require 1 placebo-controlled trialExpect to be able to reference all preclinical (toxicology), pharmacokinetic and clinical safety data of each approved drug componentPrimary endpoint of placebo-controlled trial will be side effectsEstimated total budget to NDA filing of ~$7.2 MM Task Year 1 Year 2 Year 3 Pre-IND Mtg IND Filing POC Ph1 Study Manuf Pilot and Registrational Tab 12-mon stability 12 wk & Long-term tox Pivotal trial Long term ext.trial Other NDA-enabling trials NDA Prep - Filing
GTP-011 for Motion SicknessClear Medical Need for Better Treatments Prevalence 9-38% of population1 (depending on the study) Children 2 to 12 years old are most susceptible.Novartis’ Transderm Scop (scopolamine transdermal patch) is first-line treatmentDeleterious side effects, especially in elderly, are a concernNot indicated for childrenGTP-011 potentially avoids deleterious effects of scopolamineHas been shown not to affect cognition and memory in healthy volunteers and it does not cause Alzheimer-like symptoms in the elderlyRepurposed drug with new formulationPatents filed in early 2017 Source: 1Koslucher F, Haaland E, Malsch A, Webeler J, Stoffregen TA. “Sex Differences in the Incidence of Motion Sickness Induced by Linear Visual Oscillation”. Aerosp Med Hum Perform 2015; Sep;86(9):787-93. doi: 10.3357/AMHP.4243.2015.
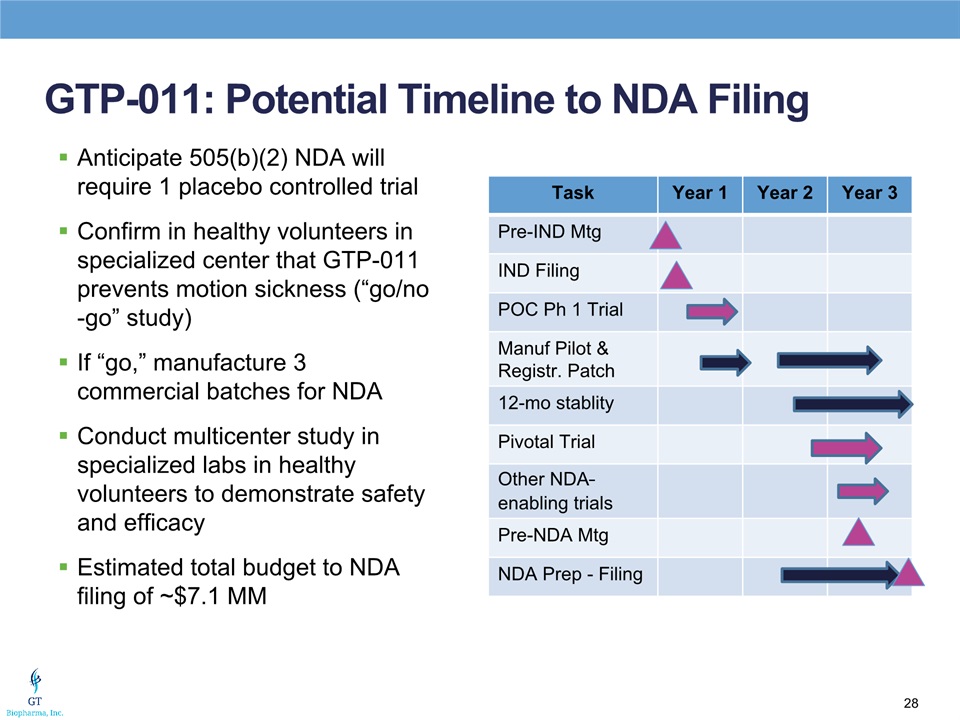
GTP-011: Potential Timeline to NDA Filing Anticipate 505(b)(2) NDA will require 1 placebo controlled trialConfirm in healthy volunteers in specialized center that GTP-011 prevents motion sickness (“go/no-go” study)If “go,” manufacture 3 commercial batches for NDAConduct multicenter study in specialized labs in healthy volunteers to demonstrate safety and efficacyEstimated total budget to NDA filing of ~$7.1 MM Task Year 1 Year 2 Year 3 Pre-IND Mtg IND Filing POC Ph 1 Trial Manuf Pilot & Registr. Patch 12-mo stablity Pivotal Trial Other NDA–enabling trials Pre-NDA Mtg NDA Prep - Filing

Anticipated Upcoming Milestones Q4 2017 – Q1 2018 GTP-004 File INDComplete Phase I Proof-of-Concept GTP-011 File INDComplete Phase I Proof-of-Concept OXS-1550 Phase II ongoing OXS-3550 IND Opening Start Phase I/II OXS-1615 Solid Tumor TriKE Start Phase 1/11 Fall OXS-4235 Start IND-enabling studies
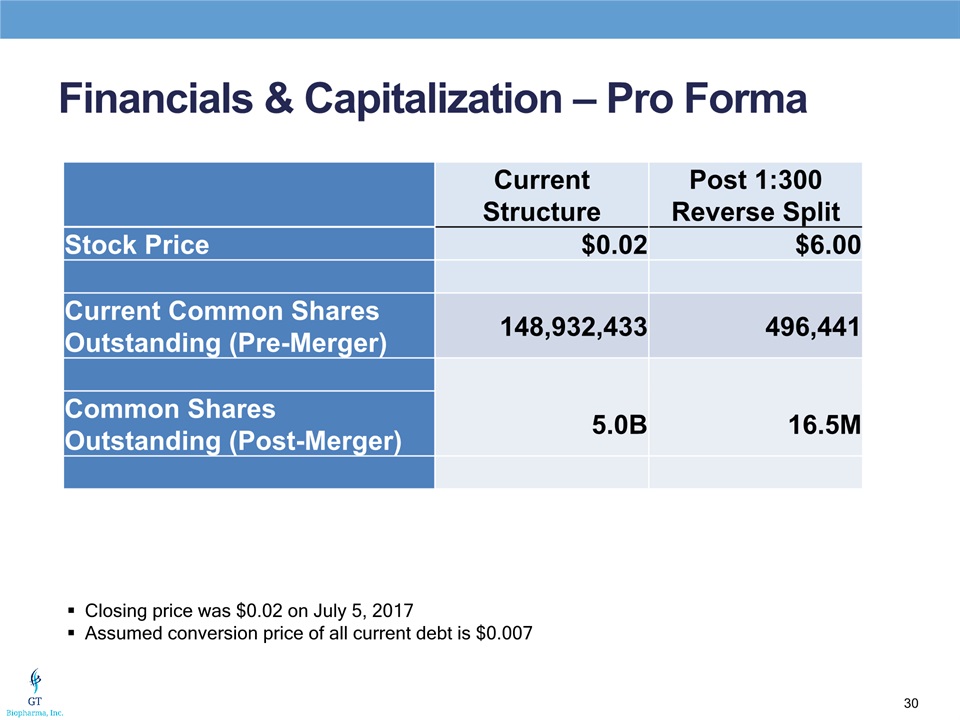
Financials & Capitalization – Pro Forma Closing price was $0.02 on July 5, 2017Assumed conversion price of all current debt is $0.007 Current Structure Post 1:300 Reverse Split Stock Price $0.02 $6.00 Current Common Shares Outstanding (Pre-Merger) 148,932,433 496,441 Common Shares Outstanding (Post-Merger) 5.0B 16.5M

Investment Highlights Diversified drug development pipeline across two therapeutic categories – oncology and CNSPipeline offers more balanced risk/reward profile with potential for steady news flowLead immuno-oncology product candidate, OXS-1550, a novel bispecific antibody drug conjugate, currently in Phase 2Lead CNS product candidate, PainBrake, anticipated to be NDA-ready in ~15-18 monthsExperienced management team with track record of success in drug development and successful exits